M2780
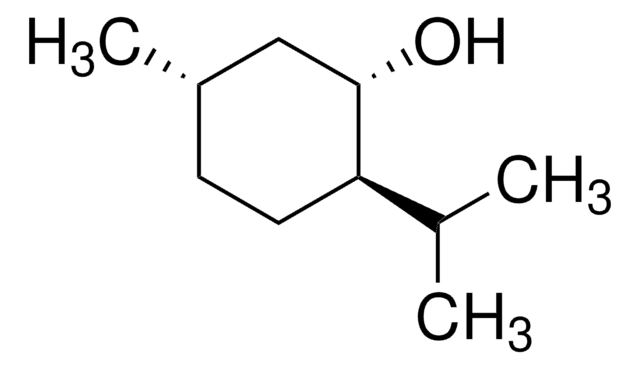
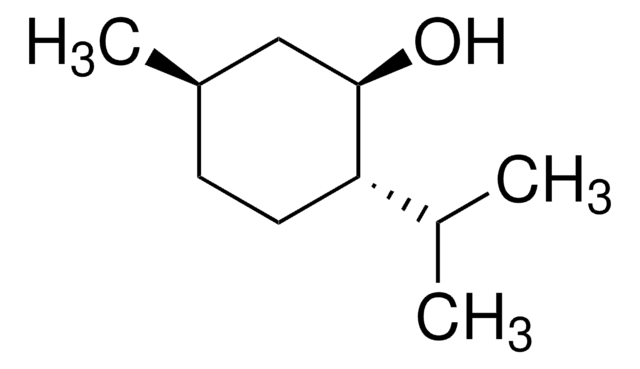
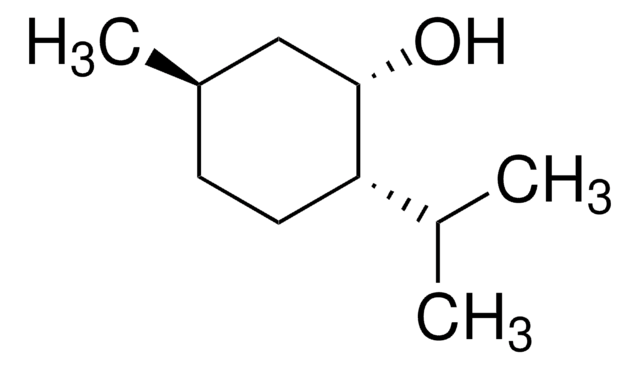
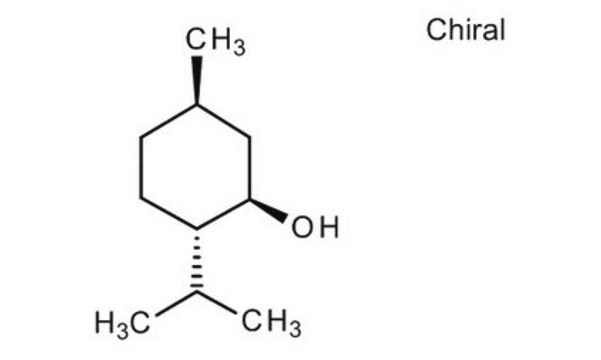
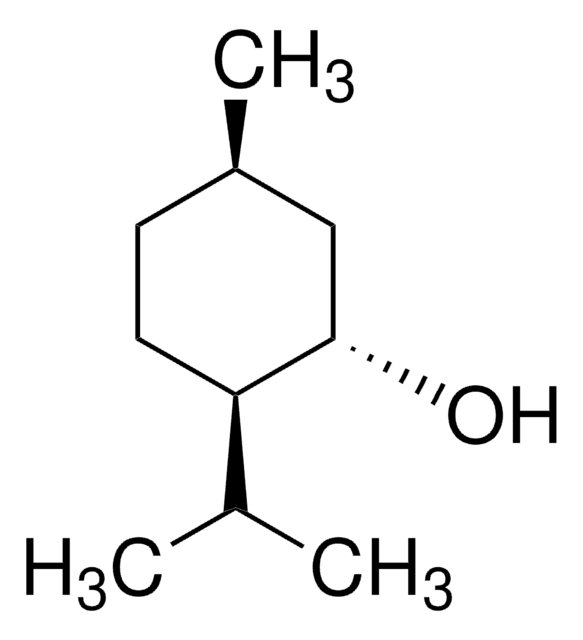
(1R,2S,5R)-(−)-Menthol
ReagentPlus®, 99%
동의어(들):
(−)-Menthol, (1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol, 5-Methyl-2-(1-methylethyl)cyclohexanol
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C10H20O
CAS Number:
Molecular Weight:
156.27
Beilstein:
1902293
EC Number:
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
생물학적 소스
synthetic (organic)
Quality Level
vapor pressure
0.8 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
99%
광학 활성
[α]20/D −50°, c = 10 in 95% ethanol
광학 순도
ee: 99% (GLC)
bp
212 °C (lit.)
mp
41-45 °C (lit.)
solubility
water: 20 mg/mL, clear, colorless
density
0.89 g/mL at 25 °C (lit.)
SMILES string
CC(C)[C@@H]1CC[C@@H](C)C[C@H]1O
InChI
1S/C10H20O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-11H,4-6H2,1-3H3/t8-,9+,10-/m1/s1
InChI key
NOOLISFMXDJSKH-KXUCPTDWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(1R,2S,5R)-(-)-Menthol is a monoterpene.
애플리케이션
(1R,2S,5R)-(-)-Menthol may be used to synthesize:
- chiral enantiopure 2-(1-hydroxyalkyl)pyridines, which can react with to form C3-symmetric tripodal ligands
- menthylphosphorodichioridite, a chiral derivatizing agent for the determination of enantiomeric purity of chiral diols or diamines by 31P NMR spectroscopy
- (2R,4S)-2,3,4,5-tetrahydro-2-(-)-menthyloxy-2-methyl-4-phenylpyrano-[3,2-c]-benzopyran-5-one, an intermediate to prepare a warfarin analog
Used to prepare reagents for chiral vinylogous Darzens and Reformatsky reactions. Important chiral auxiliary employed in the resolution of acids and for stereocontrolled synthesis.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
201.2 °F - closed cup
Flash Point (°C)
94 °C - closed cup
이미 열람한 고객
A new 31P NMR method for the enantiomeric excess determination of diols and secondary diamines with C 2 symmetry.
Brunel JM and Faure B.
Tetrahedron Asymmetry, 6(9), 2353-2356 (1995)
The Journal of Organic Chemistry, 52, 4397-4397 (1987)
Fieser, M.
Reagents for Organic Synthesis, 16, 203-203 (1992)
Marei GI, et al.
Pesticide Biochemistry and Physiology, 103(1), 56-61 (2012)
Oxindole alkaloids. A novel non-biomimetic entry to (-)-Horsfiline.
Palmisano G, et al.
Tetrahedron Asymmetry, 7(1), 1-4 (1996)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.