추천 제품
제품 라인
BioXtra
Quality Level
분석
≥98% (HPLC)
불순물
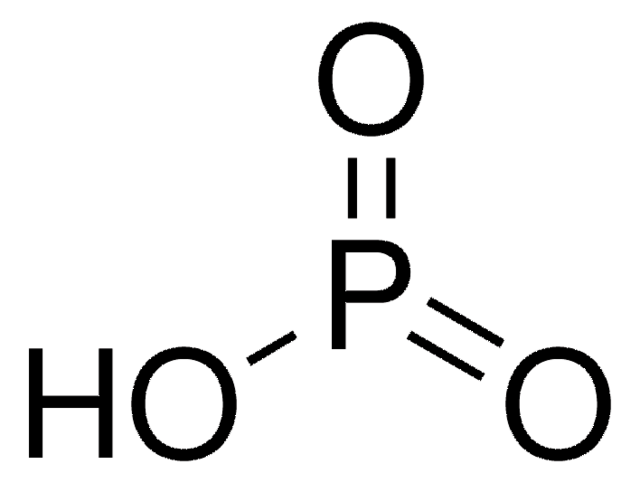
≤0.001% Phosphorus (P)
≤0.1% Insoluble matter
무기 잔류물
≤0.1%
bp
210 °C (lit.)
mp
43-46 °C (lit.)
solubility
95% ethanol: 50 mg/mL, clear to slightly hazy (colorless to faint yellow)
음이온 미량물
chloride (Cl-): ≤0.05%
sulfate (SO42-): ≤0.05%
양이온 미량물
Al: ≤0.0005%
Ca: ≤0.0005%
Cu: ≤0.0005%
Fe: ≤0.0005%
K: ≤0.005%
Mg: ≤0.0005%
Na: ≤0.005%
Pb: ≤0.001%
Zn: ≤0.0005%
저장 온도
2-8°C
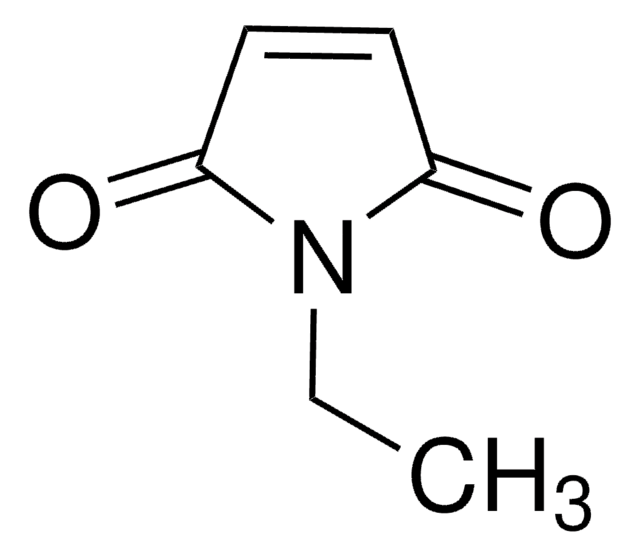
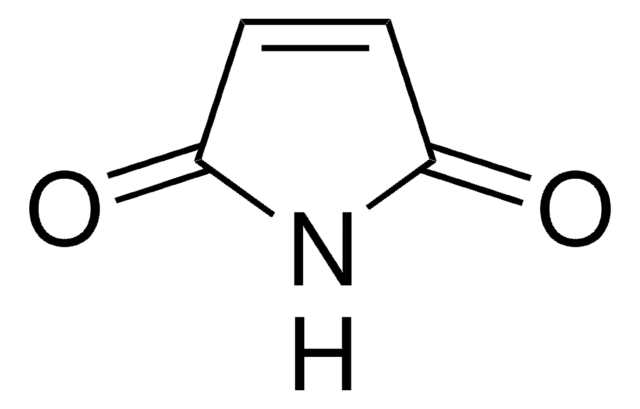
SMILES string
CCN1C(=O)C=CC1=O
InChI
1S/C6H7NO2/c1-2-7-5(8)3-4-6(7)9/h3-4H,2H2,1H3
InChI key
HDFGOPSGAURCEO-UHFFFAOYSA-N
유전자 정보
human ... SLC6A3(6531) , SLC6A4(6532)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
생화학적/생리학적 작용
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
163.2 °F - closed cup
Flash Point (°C)
72.9 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.