추천 제품
product name
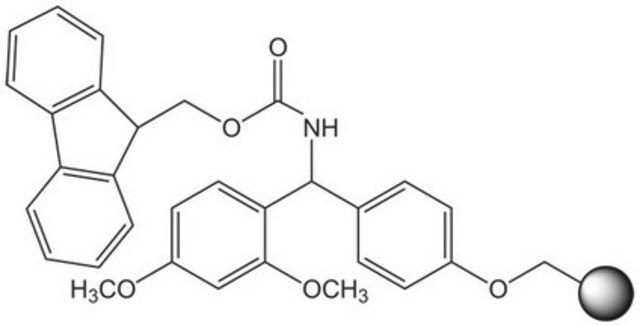
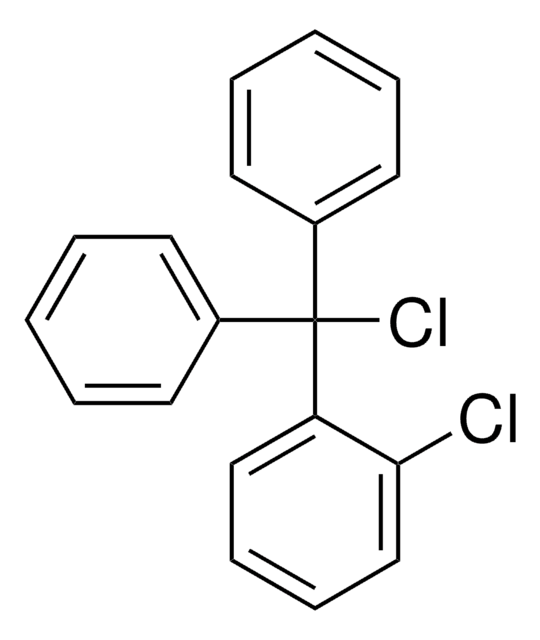
2-Chlorotrityl chloride resin (100-200 mesh), 1% DVB, Novabiochem®
Quality Level
제품 라인
Novabiochem®
형태
beads
반응 적합성
reaction type: Fmoc solid-phase peptide synthesis
제조업체/상표
Novabiochem®
응용 분야
peptide synthesis
저장 온도
2-30°C
일반 설명
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis ResinsLiterature references
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3943.
[3] K. Barlos, et al. (1991) Angew. Chem. Int. Ed. Engl., 30, 590.
[4] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 37, 513.
[5] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 562.
[6] K. Barlos, et al. (1991) Int. J. Peptide Protein Res., 38, 555.
[7] H. Wenschuh, et al. in ′Peptides 1994, Proc. 23rd European Peptide Symposium′, H. Maia (Eds), ESCOM, Leiden, 1995, pp. 287.
[8] H. Wenschuh, et al. (1995) J. Org. Chem., 60, 405.
[9] B. B. Shankar, et al. (1998) Tetrahedron Lett., 39, 2447.
[10] Z. Zhu & B. Mckittrick (1998) Tetrahedron Lett., 39, 7479.
[11] U. Heinelt, et al. (2001) Bioorg. Med. Chem. Lett., 11, 227.
[12] K. J. Elgie, et al. (2000) Tetrahedron Lett., 41, 2753.
[13] S. Batra, et al. (2000) Tetrahedron Lett., 41, 5971.
[14] M. Cardno, et al. (1995) J. Chem. Soc., Chem. Commun., 2163.
[15] I. A. Nash, et al. (1996) Tetrahedron Lett., 37, 2625.
[16] W. J. Hoekstra, et al. (1997) Tetrahedron Lett., 38, 2629.
[17] J. Perumattan, et al. (1998) Mol. Div., 3, 121.
[18] J. J. McNally, et al. (1998) Tetrahedron Lett., 39,967.
[19] M. A. Youngman & S. L. Dax (1997) Tetrahedron Lett., 38, 6347.
[20] A. Bernhardt, et al. (1997) J. Peptide Res., 50, 143.
[21] S. L. Mellor, et al. (1997) Tetrahedron Lett., 38, 3311.
[22] M. M. Meloni & M. Taddei (2001) Org. Lett., 3, 337.
[23] R. Bollhagen, et al. (1994) J. Chem. Soc ., Chem. Commun., 2559.
애플리케이션
- Efficient Synthesis of Protein Mimics by Sequential Native Chemical Ligation: This study utilized 2-Chlorotrityl chloride resin (100-200 mesh, 1% DVB) for solid-phase peptide synthesis, highlighting its effective use in complex peptide assembly processes. (Kruijtzer & Liskamp).
결합
분석 메모
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Ala-Leu loaded resin): 1.00 - 1.80 mmol/g
Swelling Volume (in CH₂Cl₂): 2.0 - 5.0 ml/g
Total swelling volume acc. Houben Weyl (in CH2Cl2): ≥ 4.2 ml/g
The polymer matrix is copoly (styrene-1 % DVB) 100 - 200 mesh.
법적 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
문서
Novabiochem® has one of the most extensive ranges of linkers and derivatized resins for Fmoc solid phase peptide synthesis. These resins have varied properties with special protocols for loading and cleaving.
Novabiochem® has one of the most extensive ranges of linkers and derivatized resins for Fmoc solid phase peptide synthesis. These resins have varied properties with special protocols for loading and cleaving.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.