추천 제품
vapor density
2.94 (vs air)
3 (vs air)
Quality Level
vapor pressure
23 mmHg ( 20 °C)
23 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
99%
불순물
≤0.5% water (Karl Fischer)
refractive index
n20/D 1.452 (lit.)
bp
106 °C (lit.)
mp
−13 °C (lit.)
solubility
organic solvents: soluble(lit.)
water: miscible(lit.)
density
0.862 g/mL at 20 °C (lit.)
SMILES string
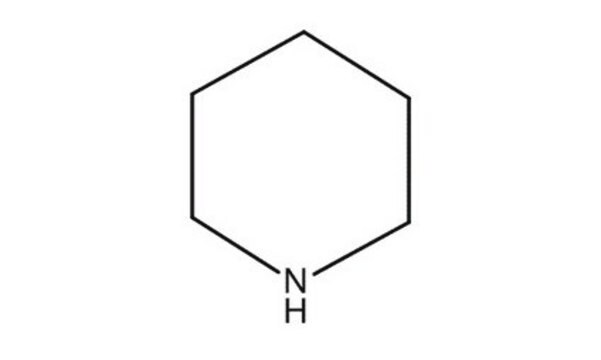
C1CCNCC1
InChI
1S/C5H11N/c1-2-4-6-5-3-1/h6H,1-5H2
InChI key
NQRYJNQNLNOLGT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Piperidine can be prepared either by nickel catalyzed hydrogenation of pyridine or by cobalt catalyzed hydrogenolysis of tetrahydrofurylamine It forms adducts with α,β,γ,δ-tetraphenylporphyriniron(II) and protoporphyriniron(II). Mossbauer spectra of these adducts have been evaluated. It constitutes the skeleton of various alkaloids. Its chemical reactivity has been discussed.
애플리케이션
Piperidine may be employed as protective and structure-directing agent in the post synthesis of MCM (Mobil Composition of Matter)-49 zeolites.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
60.8 °F - closed cup
Flash Point (°C)
16 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Rubiralta M, et al.
Piperidine: Structure, Preparation, Reactivity, and Synthetic Applications of Piperidine and its Derivatives, 43, 14-14 (2013)
Rubiralta M, et al.
Piperidine: Structure, Preparation, Reactivity, and Synthetic Applications of Piperidine and its Derivatives, 43, 3-3 (2013)
Rubiralta M, et al.
Piperidine: Structure, Preparation, Reactivity, and Synthetic Applications of Piperidine and its Derivatives, 43, 2-2 (2013)
Alma Andersson et al.
Nature communications, 12(1), 6012-6012 (2021-10-16)
In the past decades, transcriptomic studies have revolutionized cancer treatment and diagnosis. However, tumor sequencing strategies typically result in loss of spatial information, critical to understand cell interactions and their functional relevance. To address this, we investigate spatial gene expression
Moessbauer spectra of some porphyrin complexes with pyridine, piperidine, and imidazole.
Epstein LM, et al.
Inorganic Chemistry, 6(9), 1720-1724 (1967)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.