추천 제품
Grade
certified reference material
형태
liquid
특징
SNAP-N-SPIKE®, SNAP-N-SHOOT®
포장
ampule of 1 mL
제조업체/상표
Cerilliant®
농도
1.0 mg/mL in acetonitrile
기술
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
응용 분야
clinical testing
형식
single component solution
저장 온도
2-8°C
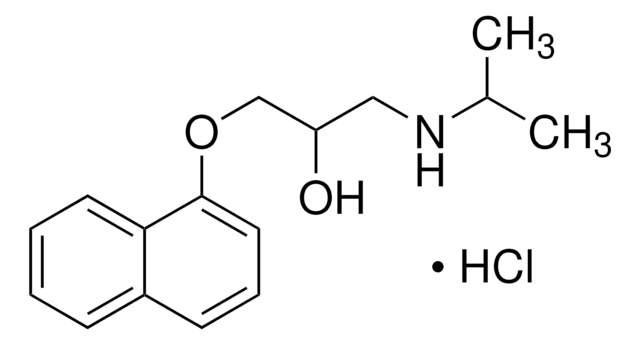
SMILES string
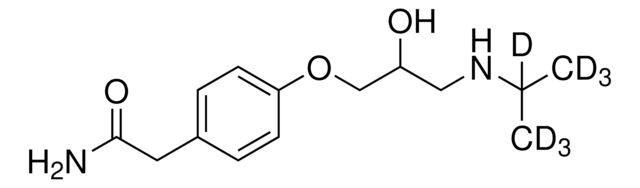
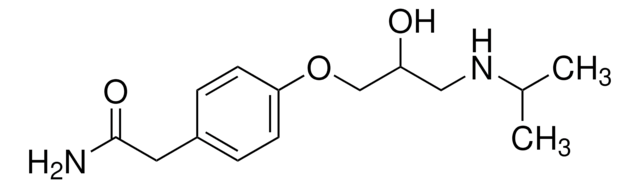
CC(C)NCC(O)COc1ccc(CC(N)=O)cc1
InChI
1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)
InChI key
METKIMKYRPQLGS-UHFFFAOYSA-N
유전자 정보
human ... ADRB1(153)
일반 설명
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.