M29800
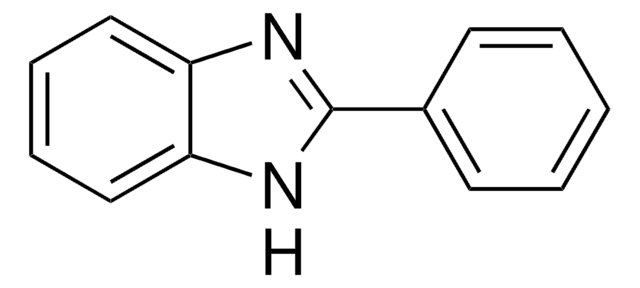
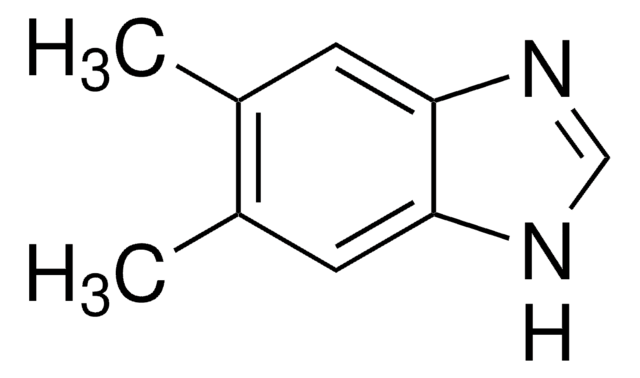
2-Methylbenzimidazole
98%
동의어(들):
2-Methyl-1H-1,3-benzodiazole, 2-Methyl-1H-benzimidazole, 2-Methyl-1H-benzo[d]imidazole, 2-Methylbenzoimidazole
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C8H8N2
CAS Number:
Molecular Weight:
132.16
Beilstein:
112264
EC Number:
MDL number:
UNSPSC 코드:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
mp
175-177 °C (lit.)
SMILES string
Cc1nc2ccccc2[nH]1
InChI
1S/C8H8N2/c1-6-9-7-4-2-3-5-8(7)10-6/h2-5H,1H3,(H,9,10)
InChI key
LDZYRENCLPUXAX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
- 2-Methylbenzimidazole is an important pharmacophore widely used in medicinal chemistry for the synthesis of various antibacterial and antifungal agents.
- It can be used as a key precursor to synthesize substituted benzimidazo[1,2-a]quinolones.
- It can be used in the synthesis of reversible solid-to-liquid phase transition coordination polymer crystals.
- 2-Methylbenzimidazole also exhibits corrosion inhibition.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Synthesis and biological evaluation of substituted benzimidazoles.
Shah K, et al.
Journal of the Indian Chemical Society, 93, 1009-1018 (2016)
Deidré van den Berg et al.
Bioorganic & medicinal chemistry, 15(11), 3692-3702 (2007-04-10)
We have recently reported that a series of (E)-8-styrylcaffeines and (E)-2-styrylbenzimidazoles are moderate to very potent competitive inhibitors of monoamine oxidase B (MAO-B). The most potent member of the series was found to be (E)-8-(3-chlorostyryl)caffeine (CSC) with an enzyme-inhibitor dissociation
Reversible solid-to-liquid phase transition of coordination polymer crystals.
Umeyama D, et al.
Journal of the American Chemical Society, 137(2), 864-870 (2015)
Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole Derivatives.
Jain P and Tiwari M
Asian Journal of Chemistry, 29(4), 838-838 (2017)
Novel strategy for synthesis of substituted benzimidazo [1, 2-a] quinolines.
Kato J Y, et al.
Organic Letters, 15(14), 3794-3797 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.