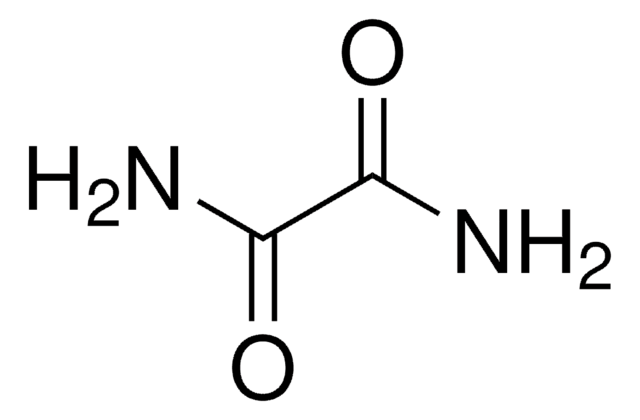
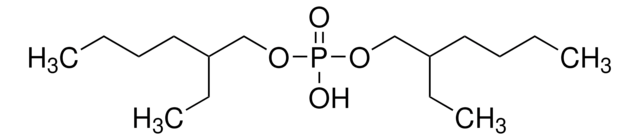
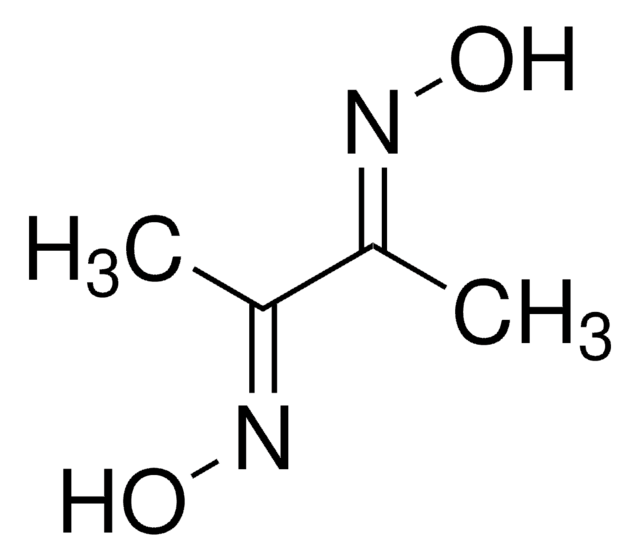
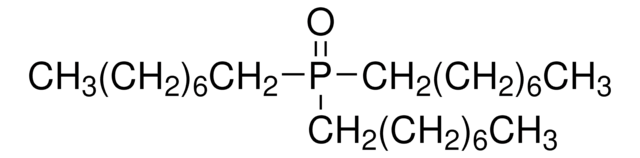
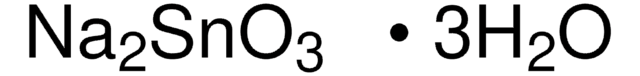추천 제품
분석
97%
양식
powder
mp
≥300 °C (lit.)
solubility
ethanol: soluble 40 mg/10 mL, clear, red
작용기
amine
SMILES string
NC(=S)C(N)=S
InChI
1S/C2H4N2S2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
InChI key
OAEGRYMCJYIXQT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dithiooxamide is reported to form complexes with Ni(II).
애플리케이션
Dithiooxamide may be used in the following studies:
- Synthesis of thiazolothiazole-linked porous organic polymers under solvothermal conditions.
- As modifier to prepare the modified glassy carbon electrode, used to investigate the electrochemical properties of quercetin, an important flavonoid derivative.
- Synthesis of new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF), used in separation and concentration of silver ions.
- Synthesis of N,N′-disubstituted dithiooxamides.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Xiang Zhu et al.
Chemical communications (Cambridge, England), 50(95), 15055-15058 (2014-10-21)
Thiazolothiazole-linked porous organic polymers have been synthesized from a facile catalyst-free condensation reaction between aldehydes and dithiooxamide under solvothermal conditions. The resultant porous frameworks exhibit a highly selective uptake of CO2 over N2 under ambient conditions.
Preparation of Dithiooxamide Derivatives.
Hurd RN, et al.
The Journal of Organic Chemistry, 26(10), 3980-3987 (1961)
Nickel (II) complexes with dithiooxamide, N, N'-di-methyl-and N, N'-di-hydroxyethyl-dithiooxamide.
Peyronel G, et al.
Inorgorganica Chimica Acta, 5, 627-633 (1971)
Ayşen Demir Mülazımoğlu et al.
Sensors (Basel, Switzerland), 12(4), 3916-3928 (2012-06-06)
Electrochemical oxidation of quercetin, as an important biological molecule, has been studied in non-aqueous media using cyclic voltammetry, electrochemical impedance spectroscopy and scanning electron microscopy. To investigate the electrochemical properties of quercetin, an important flavonoid derivative, on a different surface
Zeliyha Celik et al.
Journal of hazardous materials, 174(1-3), 556-562 (2009-10-13)
In this study, a new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF) has been synthesized by the reaction of dithiooxamide and formaldehyde. Also a well-known chelating resin of thiourea (thiooxamide)-formaldehyde (TUF) has been prepared by the reaction of thiourea and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.