추천 제품
분석
97%
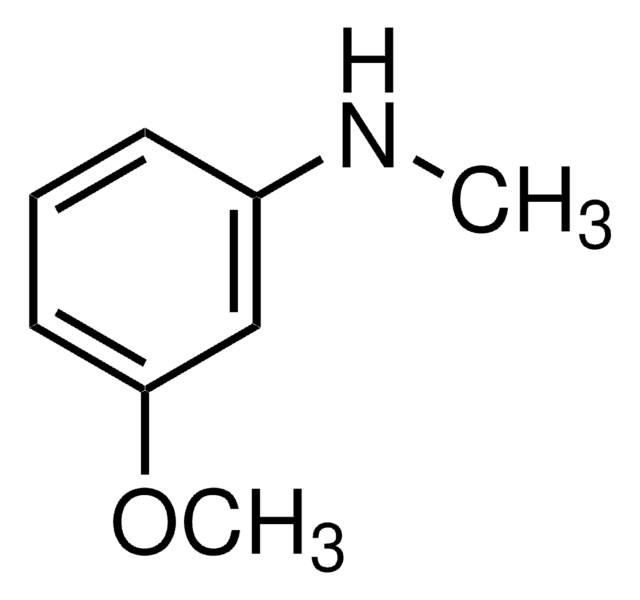
형태
liquid
refractive index
n20/D 1.581 (lit.)
bp
251 °C (lit.)
mp
−1-1 °C (lit.)
density
1.096 g/mL at 25 °C (lit.)
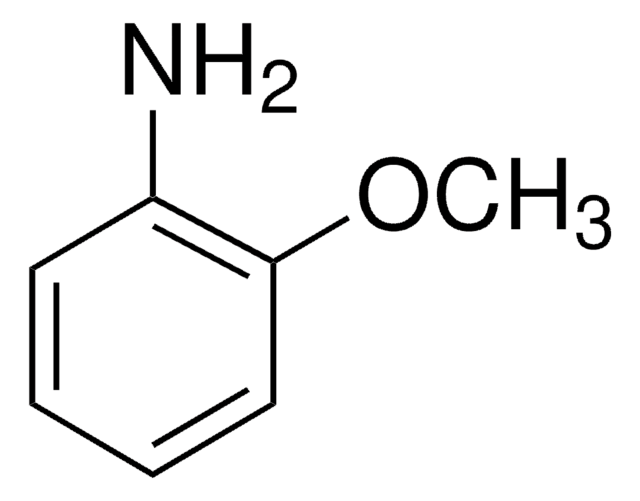
SMILES string
COc1cccc(N)c1
InChI
1S/C7H9NO/c1-9-7-4-2-3-6(8)5-7/h2-5H,8H2,1H3
InChI key
NCBZRJODKRCREW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
m-Anisidine is used in:
- The synthesis of N-substituted-3-chloro-2-azetidinones, which are potential anthelmintic agents.
- Rhodium-catalyzed synthesis of indoles and copper-catalyzed synthesis of benzimidazoles.
- In the preparation of azocalix[4]arene dyes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
258.8 °F - closed cup
Flash Point (°C)
126 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
N-Substituted-3-chloro-2-azetidinones: synthesis and characterization of novel anthelmintic agents.
Kumar M V, et al.
Research Journal of Pharmaceutical, Biological and Chemical Sciences, 1(2), 52-58 (2010)
Yasemin Bektas et al.
Scientific reports, 6, 29554-29554 (2016-07-15)
Synthetic elicitors are drug-like compounds that are structurally distinct from natural defense elicitors. They can protect plants from diseases by activating host immune responses and can serve as tools for the dissection of the plant immune system as well as
Indole synthesis via rhodium catalyzed oxidative coupling of acetanilides and internal alkynes.
Stuart D R, et al.
Journal of the American Chemical Society, 130(49), 16474-16475 (2008)
C? H Functionalization/C? N Bond Formation: Copper?Catalyzed Synthesis of Benzimidazoles from Amidines.
Brasche G and Buchwald S L
Angewandte Chemie (International Edition in English), 47(10), 1932-1934 (2008)
Azocalixarenes. 1: synthesis, characterization and investigation of the absorption spectra of substituted azocalix [4] arenes.
Karc? F, et al.
Dyes and Pigments, 59(1), 53-61 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.