180025
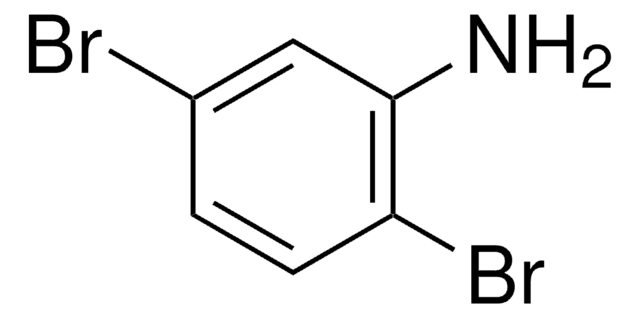
3-Bromoaniline
98%
동의어(들):
(3-Bromophenyl)amine, (m-Bromophenyl)amine, 1-Amino-3-bromobenzene, 3-Amino-1-bromobenzene, 3-Bromobenzenamine, m-Aminobromobenzene, m-Bromoaniline
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
BrC6H4NH2
CAS Number:
Molecular Weight:
172.02
Beilstein:
742028
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
양식
liquid
refractive index
n20/D 1.625 (lit.)
bp
251 °C (lit.)
mp
15-18 °C (lit.)
density
1.58 g/mL at 25 °C (lit.)
작용기
bromo
SMILES string
Nc1cccc(Br)c1
InChI
1S/C6H6BrN/c7-5-2-1-3-6(8)4-5/h1-4H,8H2
InChI key
DHYHYLGCQVVLOQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The in vitro nephrotoxic potential of 3-bromoaniline was studied.
애플리케이션
3-Bromoaniline was used in the synthesis of amino substituted quinazoline.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
>446.0 °F
Flash Point (°C)
> 230 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Cobb JM, et al.
Tetrahedron Letters, 40(5), 1045-1048 (1999)
G O Rankin et al.
Journal of applied toxicology : JAT, 15(2), 139-146 (1995-03-01)
Haloanilines are commonly used as chemical intermediates in the manufacture of a wide range of products. The purpose of this study was to examine the in vivo nephrotoxic and hepatotoxic potentials of the 3-haloanilines. The in vitro effects of the
Xiao-Feng Wu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(40), 12599-12602 (2012-09-05)
(C), its (O)K! An efficient palladium-catalyzed carbonylative synthesis of 2-alkylbenzoxazinones has been developed. By starting from 2-bromoanilines and acid anhydrides, the corresponding products were isolated in good yields.
L A Khawli et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 19(3), 297-301 (1992-04-01)
Biotinyl-m-[125I]iodoanilide (BIA) was synthesized by coupling biotin to m-[125I]iodoaniline via a mixed anhydride reaction. m-[125I]Iodoaniline was produced from the tin precursor, which was prepared using a palladium catalyzed reaction of hexabutylditin with m-bromoaniline. The radioiodinated BIA derivative is characterized by
Ali Sarafraz Yazdi et al.
Journal of chromatography. A, 1082(2), 136-142 (2005-07-23)
A novel method for the extraction of aromatic amines present in water samples is produced here coupling two-step liquid-phase microextraction with high performance liquid chromatography by using a monolithic column. The hydrophobic porous polypropylene membranes were used as the interface
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.