914088
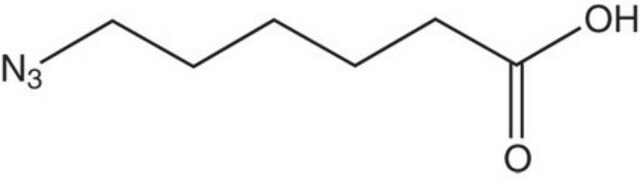
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride
≥95%
동의어(들):
(S)-2-amino-6-((2-azidoethoxy)carbonylamino)hexanoic acid hydrochloride, Clickable amino acid for bioconjugation, H-L-Lys(EO-N3)-OH HCl, Lysine-azide, UAA crosslinker
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H17N5O4 · xHCl
CAS Number:
Molecular Weight:
259.26 (free base basis)
MDL number:
UNSPSC 코드:
12352209
추천 제품
Quality Level
분석
≥95%
양식
powder
저장 온도
−20°C
SMILES string
[N+](=[N-])=NCCOC(=O)NCCCC[C@H](N)C(=O)O.C
InChI
1S/C9H17N5O4.CH4/c10-7(8(15)16)3-1-2-4-12-9(17)18-6-5-13-14-11;/h7H,1-6,10H2,(H,12,17)(H,15,16);1H4/t7-;/m0./s1
InChI key
LQERWAMRZNEGIE-FJXQXJEOSA-N
애플리케이션
N6-((2-Azidoethoxy)carbonyl)-L-lysine hydrochloride is a clickable amino acid derivative for site-specific incorporation into recombinant proteins or synthesis of chemical probes and tools for biological applications. This non-canonical lysine possesses an azide for bioorthogonal reaction with alkynes.
기타 정보
Construction of bacterial cells with an active transport system for unnatural amino acids
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
Semisynthesis of an Active Enzyme by Quantitative Click Ligation
A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast
An orthogonalized platform for genetic code expansion in both bacteria and eukaryotes
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Siqi Zheng et al.
Angewandte Chemie (International ed. in English), 53(25), 6449-6453 (2014-05-16)
Coupling the genetic code expansion technique with bioorthogonal reactions enables precise control over the conjugation site as well as the choice of fluorescent probes during protein labeling. However, the advantages of this strategy over bulky and rigid fluorescent proteins (FPs)
Chuanling Zhang et al.
Biomaterials, 80, 134-145 (2015-12-29)
Virus-based nanoparticles have shown promise as vehicles for delivering therapeutic genes. However, the rational design of viral vectors that enable selective tropism towards particular types of cells and tissues remains challenging. Here, we explored structural-functional relationships of the adeno-associated virus
Sanggil Kim et al.
Bioorganic & medicinal chemistry, 24(22), 5816-5822 (2016-10-26)
Proteins often function as complex structures in conjunction with other proteins. Because these complex structures are essential for sophisticated functions, developing protein-protein conjugates has gained research interest. In this study, site-specific protein-protein conjugation was performed by genetically incorporating an azide-containing
Michael P VanBrunt et al.
Bioconjugate chemistry, 26(11), 2249-2260 (2015-09-04)
Antibody-drug conjugates (ADC) have emerged as potent antitumor drugs that provide increased efficacy, specificity, and tolerability over chemotherapy for the treatment of cancer. ADCs generated by targeting cysteines and lysines on the antibody have shown efficacy, but these products are
Yiming Wu et al.
Bioconjugate chemistry, 27(10), 2460-2468 (2016-10-21)
Radioimmunotherapy (RIT) delivers radioisotopes to antigen-expressing cells via monoantibodies for the imaging of lesions or medical therapy. The chelates are typically conjugated to the antibody through cysteine or lysine residues, resulting in heterogeneous chelate-to-antibody ratios and various conjugation sites. To
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.