모든 사진(2)
About This Item
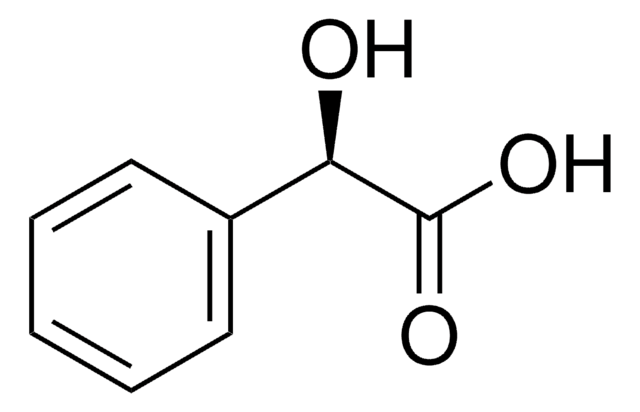
Linear Formula:
C6H5CH(OH)CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
2208678
EC Number:
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
≥99%
양식
crystals
mp
131-134 °C (lit.)
132-138 °C
작용기
carboxylic acid
hydroxyl
phenyl
SMILES string
O[C@H](C(O)=O)c1ccccc1
InChI
1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m0/s1
InChI key
IWYDHOAUDWTVEP-ZETCQYMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
(S)-(+)-Mandelic acid can be used as a starting material to synthesize (S)-cyclohexenyl phenyl glycoxilic acid, an optically active tertiary α-hydroxy acid component of (S)-oxybutynin.
It is a versatile reagent used in the resolution of racemates and the preparation of amides.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
>374.0 °F
Flash Point (°C)
> 190 °C
The Journal of Organic Chemistry, 58, 2313-2313 (1993)
Tetrahedron, 50, 5049-5049 (1994)
Chiral mandelic acid template provides a highly practical solution for (S)-oxybutynin synthesis.
Grover P T, et al.
The Journal of Organic Chemistry, 65(19), 6283-6287 (2000)
Mara Reifenrath et al.
Metabolic engineering communications, 7, e00079-e00079 (2018-10-30)
Mandelic acid is an important aromatic fine chemical and is currently mainly produced via chemical synthesis. Recently, mandelic acid production was achieved by microbial fermentations using engineered Escherichia coli and Saccharomyces cerevisiae expressing heterologous hydroxymandelate synthases (hmaS). The best-performing strains
Synthetic Communications, 23, 2761-2761 (1993)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 778052-100G | 4061832940670 |
| 778052-25G | 4061832940687 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.