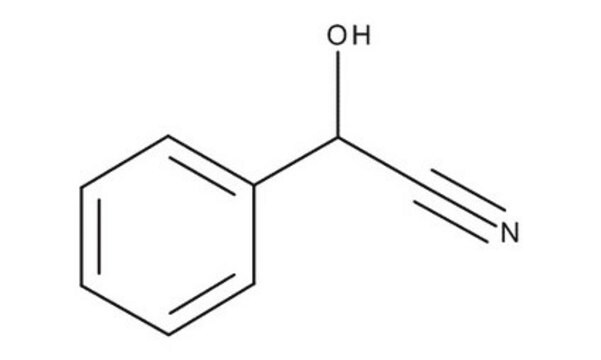
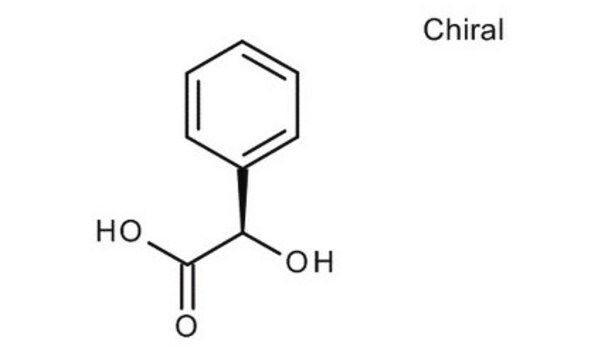
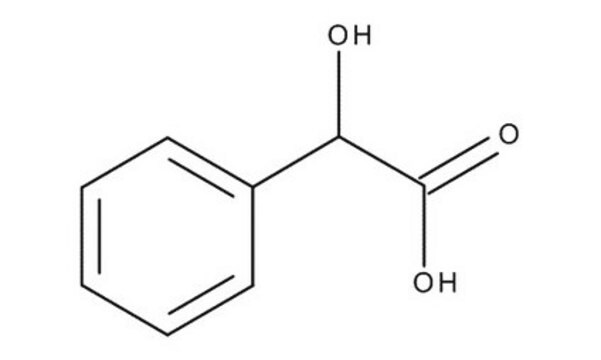
추천 제품
Grade
produced by BASF
Quality Level
분석
≥98.5%
99%
형태
crystals
광학 순도
enantiomeric excess: ≥98.5%
mp
131-133 °C (lit.)
작용기
carboxylic acid
hydroxyl
phenyl
SMILES string
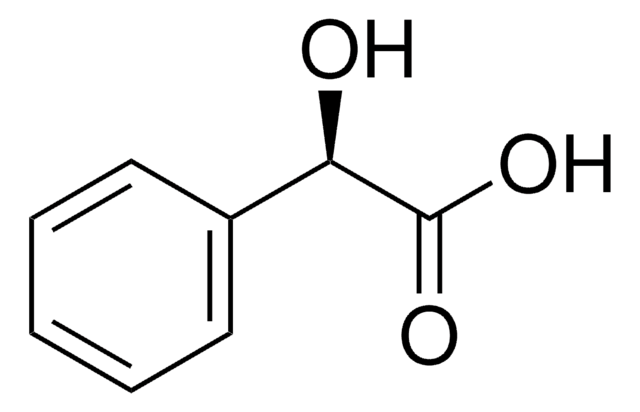
O[C@@H](C(O)=O)c1ccccc1
InChI
1S/C8H8O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7,9H,(H,10,11)/t7-/m1/s1
InChI key
IWYDHOAUDWTVEP-SSDOTTSWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(R)-(−)-Mandelic acid is a chiral resolving agent, which is also used as a chiral building block in the preparation of semisynthetic cephalosporins, antitumor agents, and penicillins.
애플리케이션
(R)-(−)-Mandelic acid can be used:
- As a chiral acid in the separation of diastereomeric salts for the synthesis of chiral pyridoindole based building block.
- In the study of chiral acid selectivity of poly(3,4-ethylenedioxythiophene) (PEDOT) based chiral conducting polymers.
법적 정보
ChiPros is a registered trademark of BASF SE
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
>374.0 °F
Flash Point (°C)
> 190 °C
가장 최신 버전 중 하나를 선택하세요:
Chiral acid selectivity displayed by PEDOT electropolymerised in the presence of chiral molecules
Sulaiman Y and Kataky R
Analyst, 137(10), 2386-2393 (2012)
Chao Ma et al.
Chirality, 23(5), 379-382 (2011-04-14)
This work reports the chiral separation of D,L-mandelic acid with cellulose membranes. Cellulose was chosen as membrane material because it possesses multichiral carbon atoms in its molecular structure unit. The flux and permselective properties of membrane using aqueous solutions of
Liang Jin et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 66(9-10), 499-506 (2011-12-24)
To study the effects of mandelic acid (MA) on the brown planthopper (BPH), Nilaparvata lugens, the survival rate and behaviour of BPH fed on an artificial diet with different dosages of MA was observed. The survival rate of BPH decreased
Zhoutong Sun et al.
Microbial cell factories, 10, 71-71 (2011-09-14)
Mandelic acid (MA), an important component in pharmaceutical syntheses, is currently produced exclusively via petrochemical processes. Growing concerns over the environment and fossil energy costs have inspired a quest to develop alternative routes to MA using renewable resources. Herein we
M Monier et al.
International journal of biological macromolecules, 55, 207-213 (2013-01-30)
An enantioselective S-mandelic acid (S-MA) imprinted chitosan (SMIC) was prepared by cross-linking of chitosan using formaldehyde cross-linker, in the presence of S-MA as an imprint template molecule and 0.5% acetic acid solution as a solvent. Non-imprinted cross-linked chitosan (NIC) as
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.