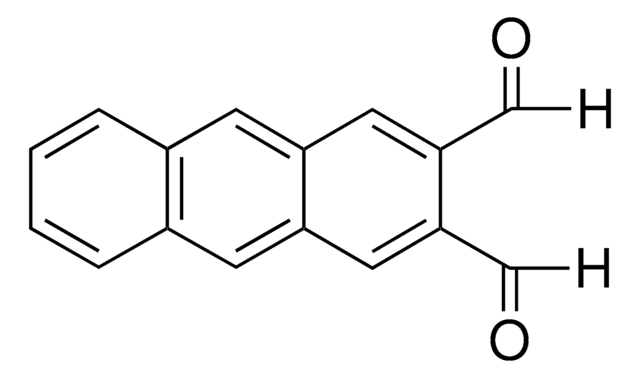
715026
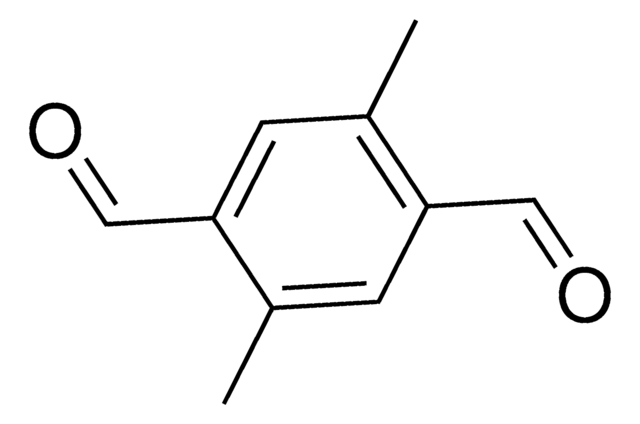
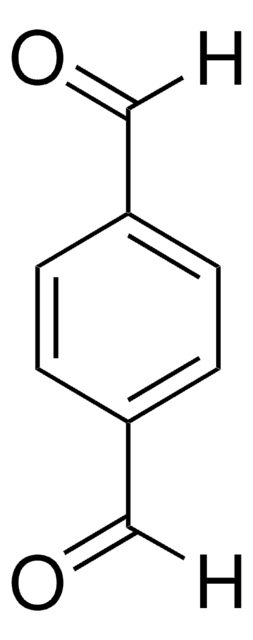
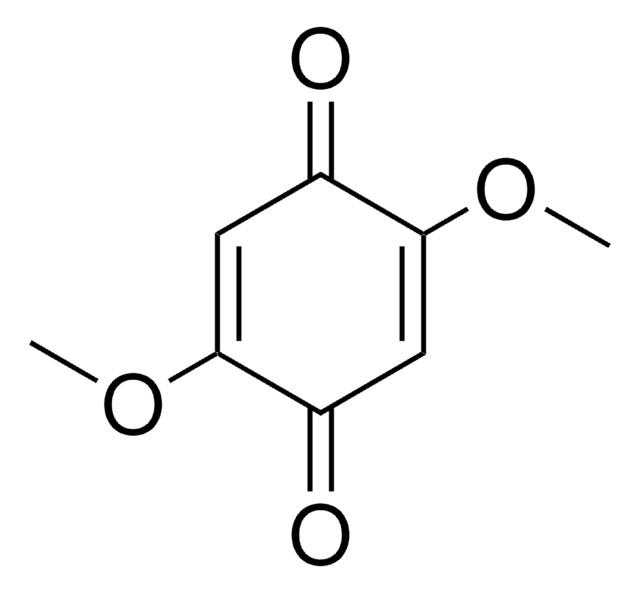
2,5-Dimethoxybenzene-1,4-dicarboxaldehyde
97%
동의어(들):
2,5-Dimethoxy-1,4-benzenedialdehyde, 2,5-Dimethoxy-1,4-benzenedicarboxaldehyde, 2,5-Dimethoxy-4-formylbenzaldehyde, 2,5-Dimethoxybenzene-1,4-dicarbaldehyde, 2,5-Dimethoxyterephthalaldehyde, 2,5-Dimethoxyterephthaldehyde
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H10O4
CAS Number:
Molecular Weight:
194.18
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
양식
solid
mp
209-213 °C
작용기
aldehyde
SMILES string
COc1cc(C=O)c(OC)cc1C=O
InChI
1S/C10H10O4/c1-13-9-3-8(6-12)10(14-2)4-7(9)5-11/h3-6H,1-2H3
InChI key
YSIIHTHHMPYKFP-UHFFFAOYSA-N
애플리케이션
2,5-Dimethoxybenzene-1,4-dicarboxaldehyde can be used as a reactant to synthesize:
- Polymeric Schiff bases using aliphatic or aromatic diamines via polycondensation reaction.
- 1,4-Bis-(α-cyano-4-methoxystyryl)-2,5-dimethoxybenzene using (4-methoxyphenyl)acetonitrile via Knoevenagel reaction in the presence of potassium t-butoxide and tetrabutylammonium hydroxide.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Elizabeth Castillo-Martínez et al.
Angewandte Chemie (International ed. in English), 53(21), 5341-5345 (2014-04-24)
The redox entity comprising two Schiff base groups attached to a phenyl ring (-N=CH-Ar-HC=N-) is reported to be active for sodium-ion storage (Ar=aromatic group). Electroactive polymeric Schiff bases were produced by reaction between non-conjugated aliphatic or conjugated aromatic diamine block
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.