모든 사진(5)
About This Item
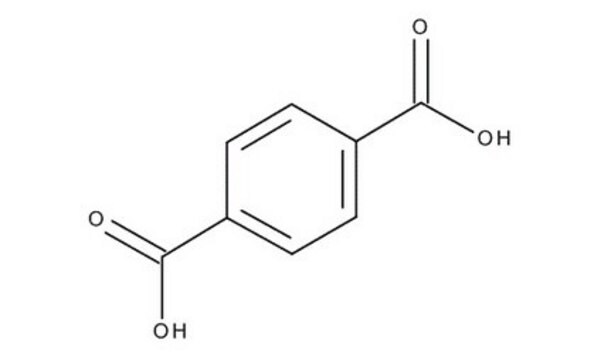
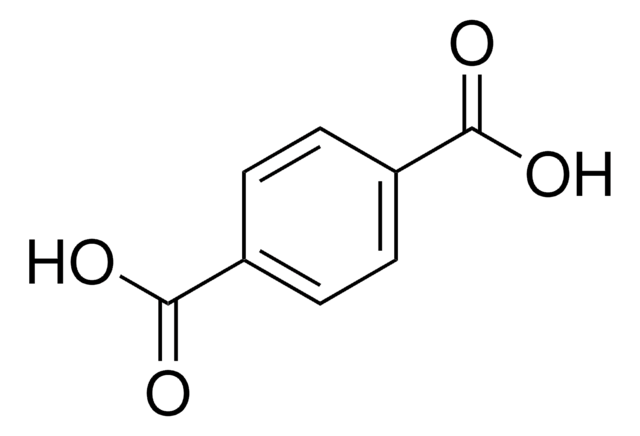
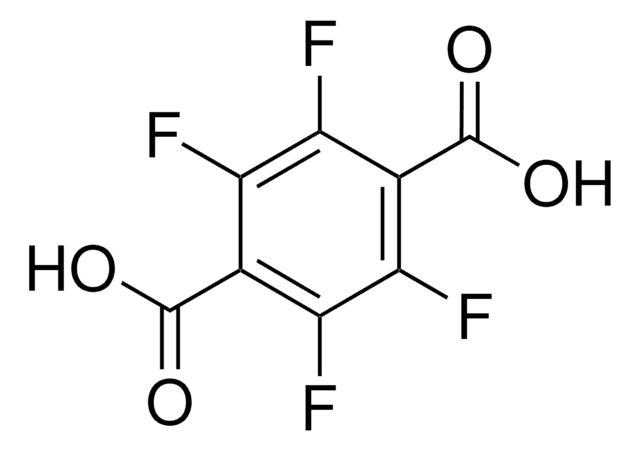
Linear Formula:
C6H4-1,4-(CO2H)2
CAS Number:
Molecular Weight:
166.13
Beilstein:
1909333
EC Number:
MDL number:
UNSPSC 코드:
12162002
eCl@ss:
39024105
PubChem Substance ID:
NACRES:
NA.23
추천 제품
vapor pressure
<0.01 mmHg ( 20 °C)
분석
98%
형태
powder
autoignition temp.
925 °F
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>300 °C (lit.)
solubility
water: ~0.017 g/L at 25 °C
density
1.58 g/cm3 at 25 °C
환경친화적 대안 카테고리
SMILES string
OC(=O)c1ccc(cc1)C(O)=O
InChI
1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
InChI key
KKEYFWRCBNTPAC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Terephthalic acid belongs to the class of monomers known as aromatic dicarboxylic acids. It is primarily used as a key monomer in the production of a high-performance polymer known as polyethylene terephthalate (PET). It is also used to prepare other polymers such as polybutylene terephthalate (PBT), polymer blends, and alloys. Terephthalic acid-based polymers are widely used in various industries including, packaging, textile fibers, polyester resins, polyurethane coatings, polyurethane foams, protective coatings, electrical components, and automotive applications due to their excellent properties such as high thermal stability, chemical resistance, lightweight, transparency, high strength, and durability. In the medical industry, terephthalic acid-based polymers may be used in the production of medical devices and equipment such as surgical sutures, tissue engineering scaffolds, and vascular grafts due to their biocompatibility, chemical resistance, and ease of processing.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
애플리케이션
Terephthalic acid can be used:
- As a monomer in the synthesis of poly(butylene terephthalate) (PBT), a type of polyester, that is used in various fields including Automotive components, textile Industry, packaging materials, electrical and electronic components.
- As an organic ligand in the synthesis of the cobalt(II) metal–organic framework (MOFs), which finds applications in electrochemical energy storage, catalysis, optoelectronics, and water treatment.
- Terephthalic acid (TPA) can be synthesized from bio-based materials for a variety of applications, which include the production of polyester fiber, non-fiber field, PET bottles, synthetic perfumes and medicines.
- Terephthalic acid is used as a linker molecule in the preparation of metal-organic frameworks (MOFs).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Photocatalytic degradation of terephthalic acid using titania and zinc oxide photocatalysts: Comparative study
Shafaei A, et al.
Desalination, 252(1-3), 8-16 (2010)
Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET
Pang J, et al.
Green Chemistry, 18(2), 342-359 (2016)
Jingqi Tian et al.
Biosensors & bioelectronics, 71, 1-6 (2015-04-17)
Considerable recent attention has been paid to homogeneous fluorescent DNA detection with the use of nanostructures as a universal "quencher", but it still remains a great challenge to develop such nanosensor with the benefits of low cost, high speed, sensitivity
Sarah E Page et al.
Environmental science & technology, 46(3), 1590-1597 (2011-12-29)
Humic acids (HAs) accept and donate electrons in many biogeochemical redox reactions at oxic/anoxic interfaces. The products of oxidation of reduced HAs by O(2) are unknown but are expected to yield reactive oxygen species, potentially including hydroxyl radical (·OH). To
Shane T Kenny et al.
Applied microbiology and biotechnology, 95(3), 623-633 (2012-05-15)
Sodium terephthalate (TA) produced from a PET pyrolysis product and waste glycerol (WG) from biodiesel manufacture were supplied to Pseudomonas putida GO16 in a fed-batch bioreactor. Six feeding strategies were employed by altering the sequence of TA and WG feeding.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.