추천 제품
분석
≥98.0%
양식
solid
반응 적합성
reaction type: click chemistry
reagent type: ligand
reaction type: Staudinger Reaction
mp
52-55 °C
작용기
phosphine
저장 온도
2-8°C
SMILES string
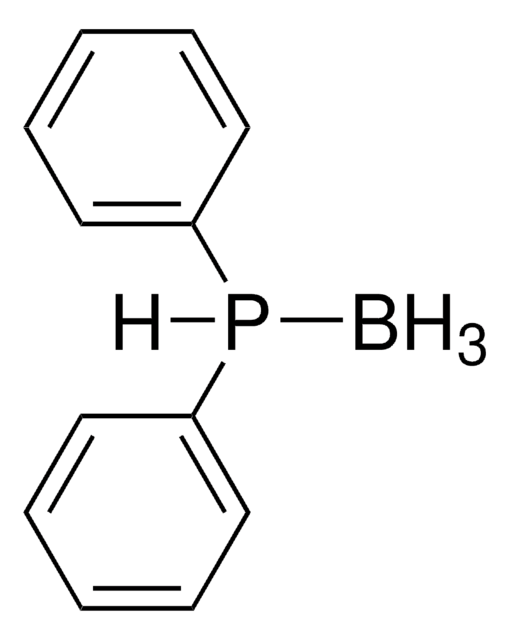
B.CC(=O)SCP(c1ccccc1)c2ccccc2
InChI
1S/C15H15OPS.BH3/c1-13(16)18-12-17(14-8-4-2-5-9-14)15-10-6-3-7-11-15;/h2-11H,12H2,1H3;1H3
InChI key
MXPNVFCCEGQGEN-UHFFFAOYSA-N
애플리케이션
- Traceless Staudinger ligation reagent with borane protecting group.
- The borane group stabilizes the phosphine against oxidation and can be easily removed with mild basic or acidic conditions to yield the active phosphine.
- After reaction with an azide, the phosphine is eliminated in the presence of water to yield a native amide bond.
- Used in the synthesis of cyclic peptides.

포장
법적 정보
관련 제품
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
문서
Based on the same working principle as the nontraceless Staudinger Ligation the auxiliary phosphine reagent can be cleaved from the product after the ligation is completed leaving a native amide bond. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a Cys residue at the ligation juncture.
The reaction between an azide and a phosphine forming an aza-ylide was discovered almost a century ago by Nobel Prize laureate Herrmann Staudinger.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.