449458
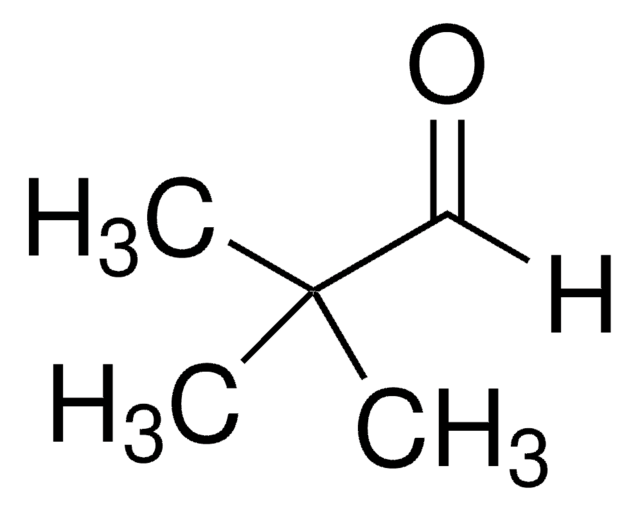
(tert-Butyldimethylsilyloxy)acetaldehyde
90%
동의어(들):
(tert-Butyldimethylsiloxy)acetaldehyde, 2-(tert-Butyldimethylsilyloxy)acetaldehyde, 2-[(tert-Butyl)dimethylsiloxy]acetaldehyde, 2-[(tert-Butyldimethylsilanyl)oxy]acetaldehyde, 2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]acetaldehyde, Dimethyl-tert-butylsilyloxyacetaldehyde
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
(CH3)3CSi(CH3)2OCH2CHO
CAS Number:
Molecular Weight:
174.31
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
90%
refractive index
n20/D 1.432 (lit.)
bp
165-167 °C (lit.)
density
0.915 g/mL at 25 °C (lit.)
작용기
aldehyde
저장 온도
2-8°C
SMILES string
CC(C)(C)[Si](C)(C)OCC=O
InChI
1S/C8H18O2Si/c1-8(2,3)11(4,5)10-7-6-9/h6H,7H2,1-5H3
InChI key
MEBFFOKESLAUSJ-UHFFFAOYSA-N
애플리케이션
(tert-Butyldimethylsilyloxy)acetaldehyde is a versatile reagent commonly used in synthetic glycobiology. It can act both as an aldol donor and an aldol acceptor in the stereocontrolled production of erythrose. It is used as an important reagent in the total synthesis of (+)-ambruticin, (−)-laulimalide, (−)-salinosporamide A, and (+)-leucascandrolide A.
Employed in the construction of the key tetrahydropyran subunit in a recent synthesis of the marine natural product (–)-dactylodide.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
140.0 °F - closed cup
Flash Point (°C)
60 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Colobert, Francoise; et al.
European Journal of Organic Chemistry, 6, 1455-1467 (2006)
Total synthesis of the microtubule-stabilizing agent (−)-Laulimalide
Paterson I, et al.
Organic Letters, 3(20), 3149-3152 (2001)
Stereocontrolled total synthesis of (+)-leucascandrolide A.
Paterson I and Tudge M
Angewandte Chemie (International Edition in English), 115(3), 357-361 (2003)
Entry to Heterocycles Based on Indium-Catalyzed Conia-Ene Reactions: Asymmetric Synthesis of (−)-Salinosporamide A.
Takahashi K, et al.
Angewandte Chemie (International Edition in English), 47(33), 6244-6246 (2008)
Total synthesis of (+)-ambruticin.
Liu P and Jacobsen EN
Journal of the American Chemical Society, 123(43), 10772-10773 (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.