418218
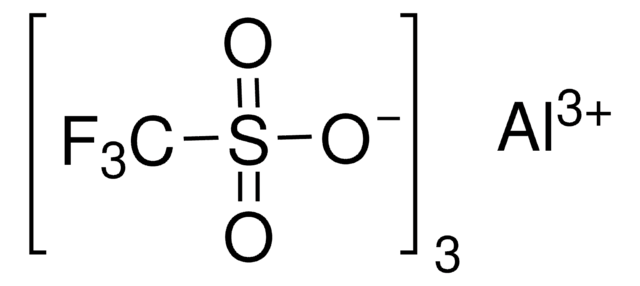
Scandium(III) triflate
99%
동의어(들):
Sc(OTf)3, Scandium(III) trifluoromethanesulfonate, Trifluoromethanesulfonic acid scandium(III) salt
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
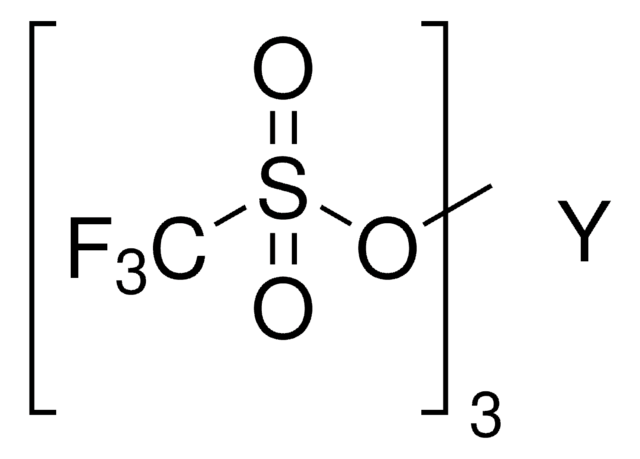
Sc(SO3CF3)3
CAS Number:
Molecular Weight:
492.16
Beilstein:
8510151
MDL number:
UNSPSC 코드:
12161600
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
powder
반응 적합성
core: scandium
reagent type: catalyst
SMILES string
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
HZXJVDYQRYYYOR-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Scandium(III) triflate is an extremely active, efficient, recoverable and reusable acylation catalyst. Its an important catalyst for the Friedel-Crafts acylation, Diels-Alder reactions and other carbon-carbon bond-forming reactions. It also stereochemically catalyzes the radical polymerization of acrylates. Scandium(III) triflate complex of (4′S,5′S)-2,6-bis[4′-(triisopropylsilyl)oxymethyl-5′-phenyl-1′,3′-oxazolin-2′-yl]pyridine has been employed as catalyst for the asymmetric Friedel-Crafts reaction between substituted indoles and methyl (E)-2-oxo-4-aryl-3-butenoates.
애플리케이션
Scandium(III) triflate was used as a catalyst in:
- Hydrothiolation reaction of aromatic and aliphatic thiols.
- Selective two-electron reduction of O2 by ferrocene derivatives.
- Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
- Synthesis of β-cyanoketones.
- Combination with triethylsilane to reductively open functionalized pyranoside rings.
- The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Kazuaki Ishihara et al.
The Journal of organic chemistry, 61(14), 4560-4567 (1996-07-12)
Scandium trifluoromethanesulfonate (triflate), which is commercially available, is a practical and useful Lewis acid catalyst for acylation of alcohols with acid anhydrides or the esterification of alcohols by carboxylic acids in the presence of p-nitrobenzoic anhydrides. The remarkably high catalytic
Krzysztof Kuciński et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(13), 4940-4943 (2015-02-18)
The first use of a Lewis acid catalyst in the addition reaction of both aromatic and aliphatic thiols to unsaturated organosilicon compounds is reported. In catalytic tests, scandium(III) triflate demonstrates high catalytic activity in this process. Under mild conditions (25 °C
Saya Kakuda et al.
Journal of the American Chemical Society, 137(9), 3330-3337 (2015-02-11)
Mononuclear copper complexes, [(tmpa)Cu(II)(CH3CN)](ClO4)2 (1, tmpa = tris(2-pyridylmethyl)amine) and [(BzQ)Cu(II)(H2O)2](ClO4)2 (2, BzQ = bis(2-quinolinylmethyl)benzylamine)], act as efficient catalysts for the selective two-electron reduction of O2 by ferrocene derivatives in the presence of scandium triflate (Sc(OTf)3) in acetone, whereas 1 catalyzes
Okamoto, Y., et al.
Macromolecular Symposia, 183, 83-83 (2002)
Jens Oelerich et al.
Organic & biomolecular chemistry, 13(9), 2793-2799 (2015-01-22)
Alkylidene malonates and α,β-unsaturated α'-hydroxyketones are demonstrated to be efficient classes of electrophiles for the scandium(III) triflate/sodium dodecyl sulphate (SDS) catalysed vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water. These substrates contain an easily removable auxiliary group that increases
문서
The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.