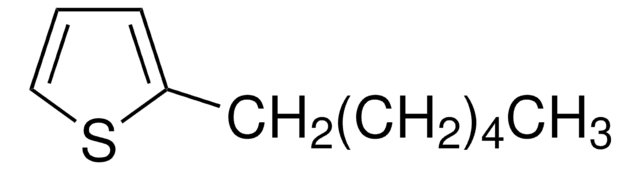모든 사진(1)
About This Item
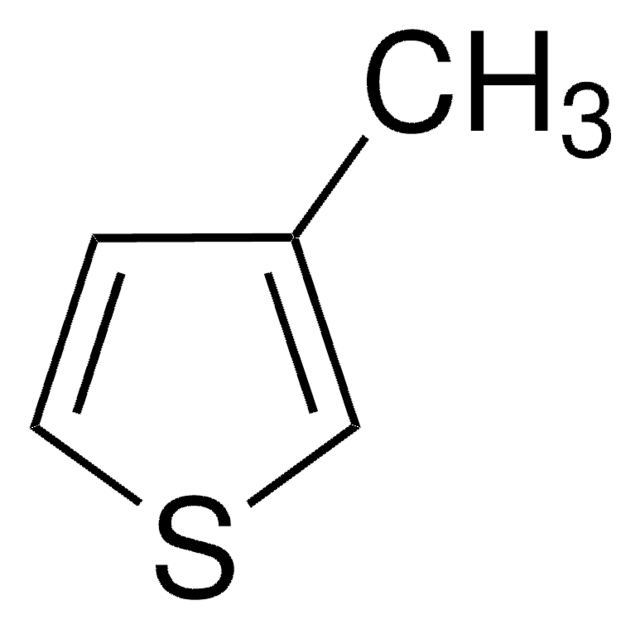
실험식(Hill 표기법):
C12H20S
CAS Number:
Molecular Weight:
196.35
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
liquid
refractive index
n20/D 1.492 (lit.)
bp
106-107 °C/3 mmHg (lit.)
density
0.92 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCc1ccsc1
InChI
1S/C12H20S/c1-2-3-4-5-6-7-8-12-9-10-13-11-12/h9-11H,2-8H2,1H3
InChI key
WQYWXQCOYRZFAV-UHFFFAOYSA-N
일반 설명
3-Octylthiophene is an alkyl thiophene derivative. It has been synthesized by the reaction of 3-bromothiophene with octylmagnesium bromide.
애플리케이션
3-Octylthiophene may be used as a starting material in the synthesis of the following:
- 2,5-dibromo-3-octylthiophene
- poly(3-butylthiophene)-b-poly(3-octylthiophene), a diblock copoly(3-alkylthiophene)
- regioregular poly(3-octylthiophene) (POT)
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Crystalline diblock conjugated copolymers: Synthesis, self-assembly, and microphase separation of poly (3-butylthiophene)-b-poly (3-octylthiophene).
Wu PT, et al.
Macromolecules, 42(7), 2317-2320 (2009)
Soluble Phenanthrenyl-Imidazole-Presenting Regioregular Poly (3-octylthiophene) Copolymers Having Tunable Bandgaps for Solar Cell Applications.
Chang YT, et al.
Advances in Functional Materials, 17(16), 3326-3331 (2007)
New convenient synthesis of highly regioregular poly (3-octylthiophene) based on the Suzuki coupling reaction.
Guillerez S and Bidan G.
Synthetic Metals, 93(2), 123-126 (1998)
Nannan Jian et al.
Physical chemistry chemical physics : PCCP, 21(13), 7174-7182 (2019-03-20)
Conjugated fluorophores have been extensively used for fluorescence sensing of various substances in the field of life processes and environmental science, due to their noninvasiveness, sensitivity, simplicity and rapidity. Most existing conjugated fluorophores exhibit excellent light-emitting performance in dilute solutions
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)