196282
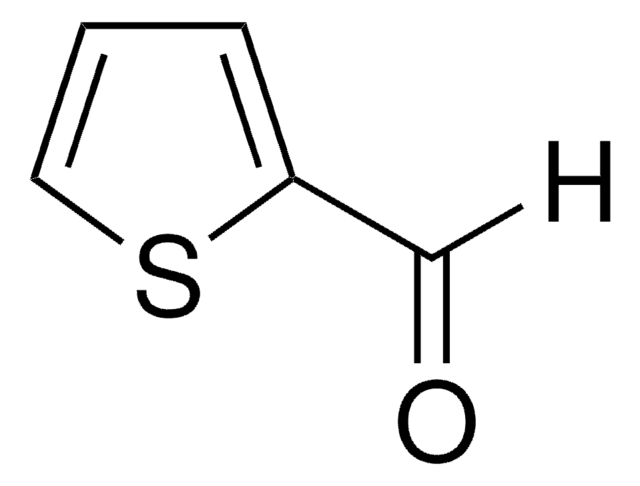
3-Thiophenecarboxaldehyde
98%
동의어(들):
3-Formylthiophene, 3-Thienaldehyde, 3-Thienylcarboxaldehyde, 3-Thiophenealdehyde, 3-Thiophenecarbaldehyde, Thiofuran-3-carboxaldehyde, Thiophen-3-aldehyde
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C5H4OS
CAS Number:
Molecular Weight:
112.15
Beilstein:
105889
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
0.31 mmHg ( 20 °C)
Quality Level
분석
98%
양식
liquid
autoignition temp.
>392 °F
refractive index
n20/D 1.583 (lit.)
bp
194-196 °C (lit.)
86-87 °C/20 mmHg (lit.)
density
1.28 g/mL at 25 °C (lit.)
작용기
aldehyde
저장 온도
2-8°C
SMILES string
[H]C(=O)c1ccsc1
InChI
1S/C5H4OS/c6-3-5-1-2-7-4-5/h1-4H
InChI key
RBIGKSZIQCTIJF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
3-Thiophenecarboxaldehyde has been used in the synthesis of:
- series of 4-substituted 2-thiophenesulfonamides
- acetal and ketal derivatives of 4′-demethylepipodophyllotoxin-β-D-glucoside and epipodophyllotoxin-β-D-glucoside
- 1,2-di-3-thienyl-2-hydroxyethanone (3,3′-thenoin), 3-thienyl symmetric analog of benzoin
J M Holmes et al.
Journal of medicinal chemistry, 37(11), 1646-1651 (1994-05-27)
A series of 4-substituted 2-thiophenesulfonamides was prepared from 3-thiophenecarboxaldehyde using metalation chemistry developed for 3-furaldehyde. Several of these compounds inhibit carbonic anhydrase II in vitro at concentrations of less than 10 nM. In addition, none of these compounds exhibit sensitization
1, 2-Di-3-thienyl-2-hydroxyethanone (3, 3′-thenoin).
Crundwell G, et al.
Acta Crystallographica Section E, Structure Reports Online, 58(6), o668-o670 (2002)
R S Gupta et al.
Anti-cancer drug design, 2(1), 1-12 (1987-08-01)
We have synthesized acetal and ketal derivatives of 4'-demethylepipodophyllotoxin-beta-D-glucoside (DMEPG) and epipodophyllotoxin-beta-D-glucoside (EPG) with a number of different aldehydes (viz. acetaldehyde, propionaldehyde, 2-thiophenecarboxaldehyde, 3-thiophenecarboxaldehyde, 2-furancarboxaldehyde, benzaldehyde, phenylacetaldehyde, hydrocinnamaldehyde) and acetone. The cross resistance of these compounds towards a set of
Kyungsil Yoon et al.
Cells, 8(3) (2019-03-15)
Chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is an orphan receptor and member of the nuclear receptor superfamily. Among a series of methylene substituted diindolylmethanes (C-DIMs) containing substituted phenyl and heteroaromatic groups, we identified 1,1-bis(3'-indolyl)-1-(4-pyridyl)-methane (DIM-C-Pyr-4) as an activator of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 196282-1G | 4061838096401 |
| 196282-10G | 4061838762009 |
| 196282-50G | |
| 196282-5G | 4061838089960 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.