About This Item
추천 제품
Quality Level
분석
≥95%
광학 활성
[α]22/D +336°, c = 1.2 in methanol
mp
178 °C (dec.) (lit.)
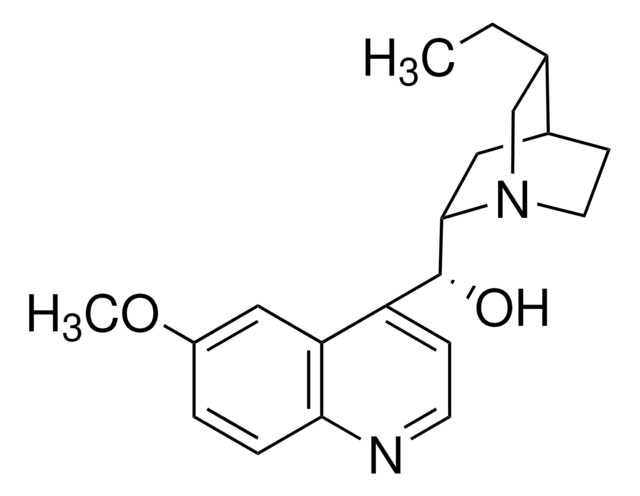
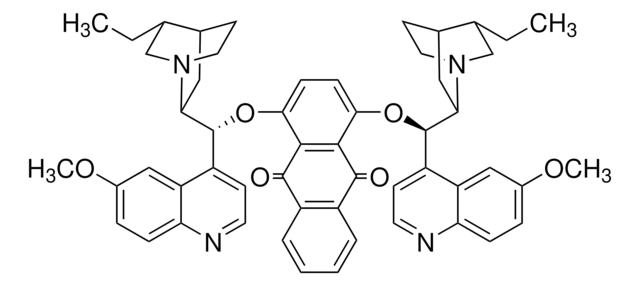
SMILES string
CC[C@@H]1CN2CCC1CC2[C@H](Oc3nnc(O[C@@H](C4CC5CCN4C[C@@H]5CC)c6ccnc7ccc(OC)cc67)c8ccccc38)c9ccnc%10ccc(OC)cc9%10
InChI
1S/C48H54N6O4/c1-5-29-27-53-21-17-31(29)23-43(53)45(35-15-19-49-41-13-11-33(55-3)25-39(35)41)57-47-37-9-7-8-10-38(37)48(52-51-47)58-46(44-24-32-18-22-54(44)28-30(32)6-2)36-16-20-50-42-14-12-34(56-4)26-40(36)42/h7-16,19-20,25-26,29-32,43-46H,5-6,17-18,21-24,27-28H2,1-4H3/t29-,30+,31-,32+,43-,44-,45-,46-/m1/s1
InChI key
YUCBLVFHJWOYDN-PPIALRKJSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- As a catalyst Asymmetric and chemoselective N-allylic alkylation of indoles with Morita-Baylis-Hillman carbonates to form pyrrolo[1,2-a]indole and pyrrolo[3,2,1-ij]quinoline derivatives.
- As a ligand for the osmium catalyzed-Sharpless asymmetric dihydroxylation step of (S)-a-benzoyloxy carboxylic acids multistep synthesis.
- As a ligand for the carbamate based asymmetric aminohydroxylation of styrene derivatives to form N-carbamate protected R-arylglycinols.
물리적 형태
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type N95 (US)
이미 열람한 고객
문서
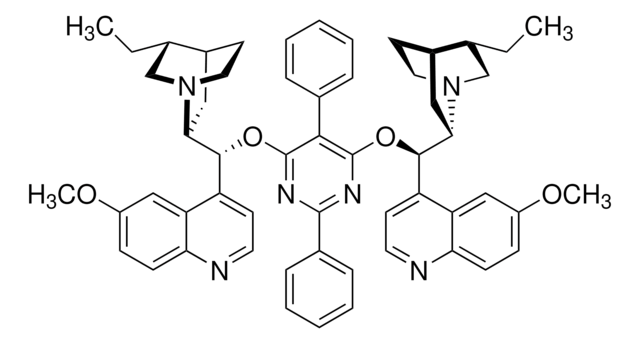
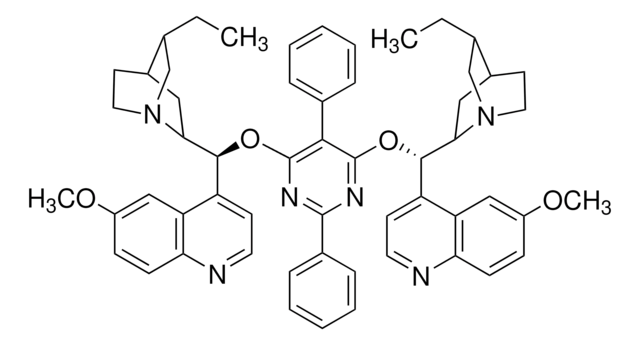
Asymmetric phase-transfer catalysis (PTC) has been recognized as a “green” alternative to many homogeneous synthetic organic transformations, and has found widespread application. Synthetically modified cinchona alkaloids are typical chiral organocatalysts used in asymmetric PTC.
Asymmetric phase-transfer catalysis (PTC) has been recognized as a “green” alternative to many homogeneous synthetic organic transformations, and has found widespread application. Synthetically modified cinchona alkaloids are typical chiral organocatalysts used in asymmetric PTC.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.


![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)