추천 제품
Quality Level
분석
≥97.0% (CHN)
양식
solid
mp
85-89 °C (lit.)
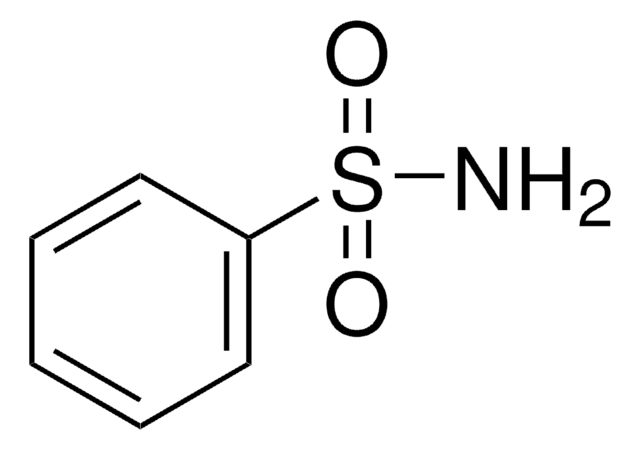
SMILES string
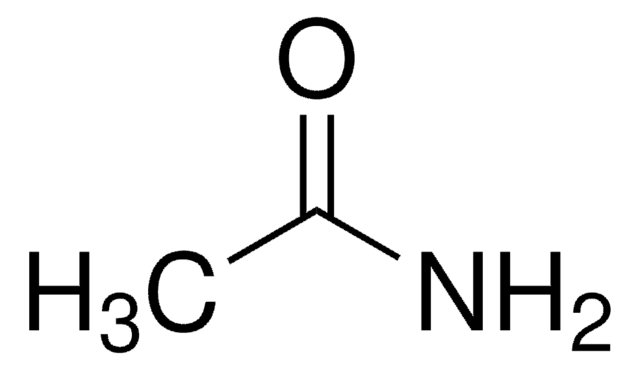
CS(N)(=O)=O
InChI
1S/CH5NO2S/c1-5(2,3)4/h1H3,(H2,2,3,4)
InChI key
HNQIVZYLYMDVSB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Methanesulfonamide is used in the synthesis of important organic reagents such as N-(2-methylthio-1-p-toluenesufonyl)methanesulfonamide and tert-butyl ((2-(trimethylsilyl)ethyl)sulfonyl)carbamate.
- It can be used as a source of nitrogen in the conversion of carboxylic acids to corresponding nitriles.
- It is widely used as a reagent in the synthesis of medicinally important compounds such as derivatives of indole-N-acetamide, methanesulfonamide pyrimidine-substituted 3,5-dihydroxy-6-heptenoates and repertaxin.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
A Convenient Synthesis of Chloronitrobenzonitrile Isomers and Homologs.
Grivsky E M
Bull. Soc. Chim. Belg., 80(3?4), 245-252 (1971)
Development and preliminary optimization of indole-N-acetamide inhibitors of hepatitis C virus NS5B polymerase.
Harper S, et al.
Journal of Medicinal Chemistry, 48(5), 1314-1317 (2005)
Methanesulfonamide.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2007)
2-Arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors.
Allegretti M, et al.
Journal of Medicinal Chemistry, 48(13), 4312-4331 (2005)
Synthesis and biological activity of methanesulfonamide pyrimidine-and N-methanesulfonyl pyrrole-substituted 3, 5-dihydroxy-6-heptenoates, a novel series of HMG-CoA reductase inhibitors.
Watanabe M, et al.
Bioorganic & Medicinal Chemistry, 5(2), 437-444 (1997)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.