37347
O-(2-Oxo-1(2H)pyridyl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate
≥99.0% (HPLC)
동의어(들):
O-(1,2-Dihydro-2-oxo-1-pyridyl)-N,N,N′-N′-tetramethyluronium tetrafluoroborate, TPTU
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H16BF4N3O2
CAS Number:
Molecular Weight:
297.06
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥99.0% (HPLC)
반응 적합성
reaction type: Coupling Reactions
mp
140 °C (dec.) (lit.)
응용 분야
peptide synthesis
작용기
amine
저장 온도
2-8°C
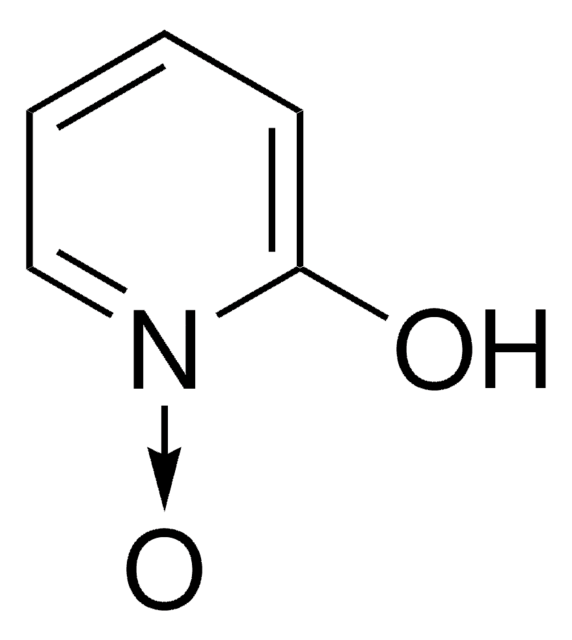
SMILES string
F[B-](F)(F)F.CN(C)C(\ON1C=CC=CC1=O)=[N+](/C)C
InChI
1S/C10H16N3O2.BF4/c1-11(2)10(12(3)4)15-13-8-6-5-7-9(13)14;2-1(3,4)5/h5-8H,1-4H3;/q+1;-1
InChI key
CZQGINAUZYECAI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reagent for:
Amidation
Peptide Coupling
Esterification of nucleosides to solid phase supports for oligonucleoside synthesis
Amidation
Peptide Coupling
Esterification of nucleosides to solid phase supports for oligonucleoside synthesis
기타 정보
Coupling reagent for peptide synthesis, especially suited for segment condensation with little racemization
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Sens. 1A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
R T Pon et al.
Bioconjugate chemistry, 10(6), 1051-1057 (1999-11-24)
Nucleosides can be esterified to solid-phase supports using uronium or phosphonium coupling reagents and a coupling additive, such as 1-hydroxybenzotriazole (HOBT), 7-aza-1-hydroxybenzotriazole (HOAT), N-methylimidazole (NMI), or 4-(dimethylamino)pyridine (DMAP). However, DMAP was far superior to other additives and high nucleoside loadings
C M Huntley et al.
Nucleosides, nucleotides & nucleic acids, 20(4-7), 731-733 (2001-09-21)
The synthesis of 1-(beta-D-ribofuranosyl)pyridin-2-one-3-carboxylic acid and the 3-carboxamide as well as a short series of 3N-carboxamides, prepared by TPTU/HOBt coupling of primary amines with 1-(beta-D-ribofuranosyl)pyridin-2-one-3-carboxylic acid, and their evaluation as anti-infective agents is described.
Tomohisa Sawada et al.
Journal of the American Chemical Society, 133(19), 7336-7339 (2011-04-28)
Artificial mimicry of α-helices offers a basis for development of protein-protein interaction antagonists. Here we report a new type of unnatural peptidic backbone, containing α-, β-, and γ-amino acid residues in an αγααβα repeat pattern, for this purpose. This unnatural
Knorr, R, et al.
Tetrahedron Letters, 30, 1927-1927 (1989)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.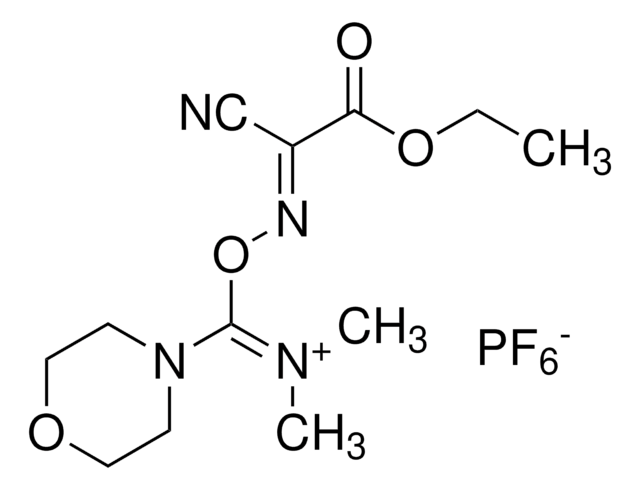




![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)