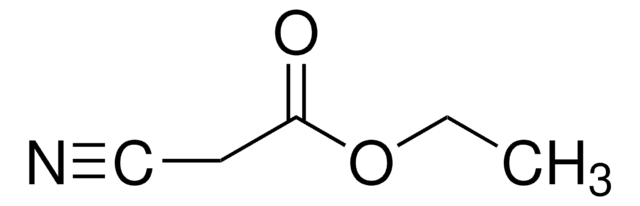
233412
Ethyl (hydroxyimino)cyanoacetate
97%, for peptide synthesis
동의어(들):
Ethyl cyano(hydroxyimino)acetate, Ethyl cyanoglyoxalate-2-oxime, Ethyl isonitrosocyanoacetate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
NCC(=NOH)CO2C2H5
CAS Number:
Molecular Weight:
142.11
Beilstein:
774783
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
제품명
Ethyl (hydroxyimino)cyanoacetate, 97%
Quality Level
분석
97%
양식
solid
mp
130-132 °C (lit.)
응용 분야
peptide synthesis
작용기
amine
ester
nitrile
oxime
SMILES string
CCOC(=O)C(=N\O)\C#N
InChI
1S/C5H6N2O3/c1-2-10-5(8)4(3-6)7-9/h9H,2H2,1H3/b7-4+
InChI key
LCFXLZAXGXOXAP-QPJJXVBHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Ethyl (hydroxyimino)cyanoacetate is a potential replacement for zobenzotriazole and benzotriazole derivatives used in peptide synthesis.
Ethyl (hydroxyimino)cyanoacetate is also called as Oxyma. It is a highly efficient greener alternative for the amide and peptide synthesis.
Ethyl (hydroxyimino)cyanoacetate is also called as Oxyma. It is a highly efficient greener alternative for the amide and peptide synthesis.
애플리케이션
Ethyl (hydroxyimino)cyanoacetate has been used as an additive for the carbodiimide-mediated amide bond formation during established peptide synthesis method.For peptide synthesis grade material, please see product 851086.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Ranko Gacesa et al.
International journal of molecular sciences, 21(20) (2020-10-16)
Siderophores are iron-complexing compounds synthesized by bacteria and fungi. They are low molecular weight compounds (500-1500 Daltons) possessing high affinity for iron(III). Since 1970 a large number of siderophores have been characterized, the majority using hydroxamate or catecholate as functional
Hangyu Zhang et al.
Acta biomaterialia, 55, 183-193 (2017-04-04)
Self-assembling peptides programed by sequence design to form predefined nanostructures are useful for a variety of biomedical applications. However, assemblies of classic ionic self-complementary peptides are unstable in neutral pH, while charged peptide hydrogels have low mechanical strength. Here, we
Daniele Maiolo et al.
ChemistryOpen, 9(2), 253-260 (2020-02-29)
Here, we demonstrate that introduction of halogen atoms at the tyrosine 10 phenol ring of the DSGYEV sequence derived from the flexible amyloid-β N-terminus, promotes its self-assembly in the solid state. In particular, we report the crystal structures of two halogen-modified
Doaa M Anwar et al.
Bioconjugate chemistry, 29(9), 3026-3041 (2018-08-16)
In this study, promising approaches of dual-targeted micelles and drug-polymer conjugation were combined to enable injection of poorly soluble anticancer drugs together with site-specific drug release. Ursodeoxycholic acid (UDCA) as a hepatoprotective agent was grafted to maltodextrin (MD) via carbodiimide
Virginia Brancato et al.
Acta biomaterialia, 57, 47-58 (2017-05-10)
Therapeutic approaches based on nanomedicine have garnered great attention in cancer research. In vitro biological models that better mimic in vivo conditions are crucial tools to more accurately predict their therapeutic efficacy in vivo. In this work, a new 3D
문서
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.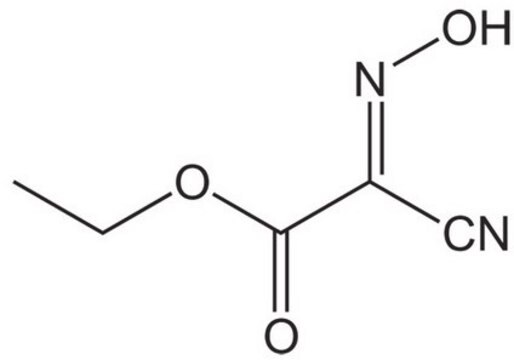


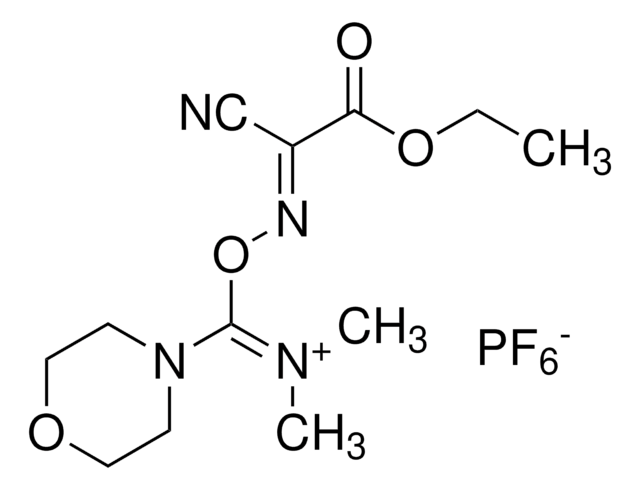



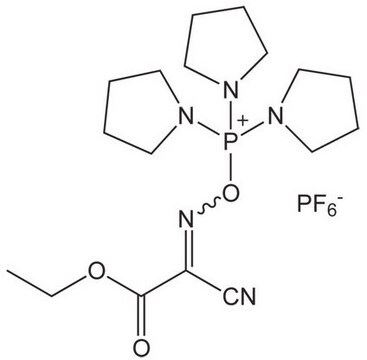
![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)