모든 사진(4)
About This Item
Linear Formula:
(CH3COO)3BHNa
CAS Number:
Molecular Weight:
211.94
Beilstein:
4047608
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
반응 적합성
reagent type: reductant
환경친화적 대안 제품 점수
old score: 5
new score: 1
Find out more about DOZN™ Scoring
환경친화적 대안 제품 특성
Waste Prevention
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
116-120 °C (dec.) (lit.)
환경친화적 대안 카테고리
SMILES string
[Na+].CC(=O)O[BH-](OC(C)=O)OC(C)=O
InChI
1S/C6H10BO6.Na/c1-4(8)11-7(12-5(2)9)13-6(3)10;/h7H,1-3H3;/q-1;+1
InChI key
HHYFEYBWNZJVFQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
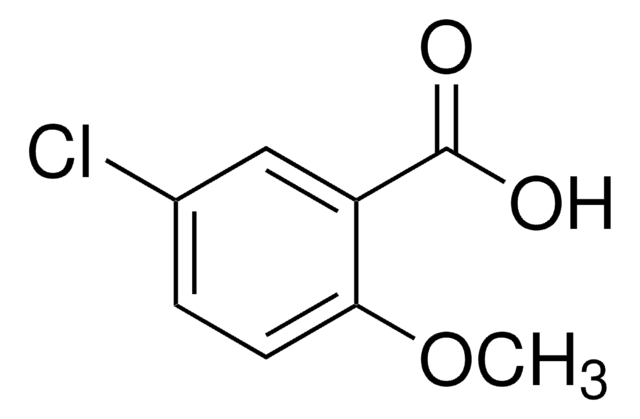
Sodium triacetoxyborohydride (NaBH(OAc)3) is particularly effective in reductive aminations due to its large scope, mildness, and selectivity. It is preferred to sodium cyanoborohydride(NaBH3CN) in many applications due to reduced toxicity of the side products formed, and better yields and reproducibility during synthesis.
We are committed to bringing you Greener Alternative Products, which adhere to one of the four categories of Greener Alternatives. This product belongs to category of Re-engineered products, showing key improvements in Green Chemistry Principles “Waste Prevention”, “Design for Energy Efficiency” and “Use of Renewable Feedstocks”. Click here to view its DOZN scorecard.
애플리케이션
Sodium triacetoxyborohydride or [NaBH(OAc)3] can be used as a reagent:
- In the reductive amination of ketones and aldehydes.
- To prepare N-benzyl-γ-valerolactam by reacting with methyl 4-oxopentanoate and benzylamine via reductive amination/lactamization.
- To reduce imines and enamines to corresponding amines.
- To reduce quinolines and isoquinolines to corresponding tetrahydro derivatives.
- In the hydroboration of alkenes.
- To synthesize nitroxide biradicals for creating high relaxivity terminal groups linkage to dendrimers.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Sol. 1 - Repr. 1B - Water-react 1
보충제 위험성
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Tetrahedron Letters, 45, 3975-3978 (2004)
Tetrahedron Letters, 31, 5595-5595 (1990)
A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes
Abdel-Magid AF and Mehrman SJ
Organic Process Research & Development, 10(5), 971-1031 (2006)
ACS Symp. Ser., 641, 201-216 (1996)
Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis
Periasamy M and Thirumalaikumar M
Journal of Organometallic Chemistry, 609(1-2), 137-151 (2000)
문서
Sodium triacetoxyborohydride
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.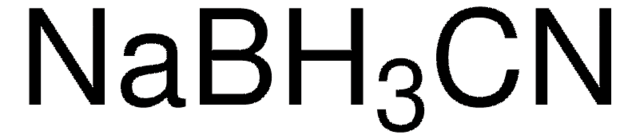



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)