모든 사진(1)
About This Item
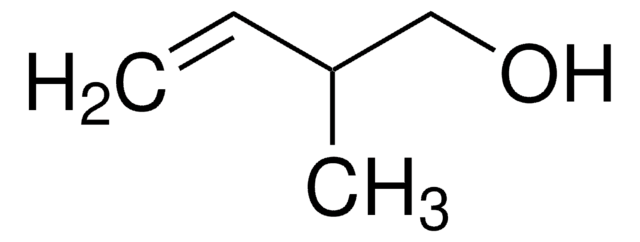
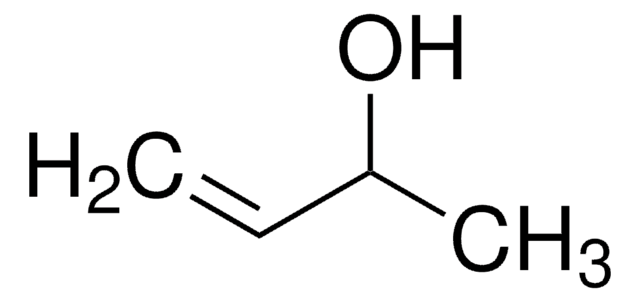
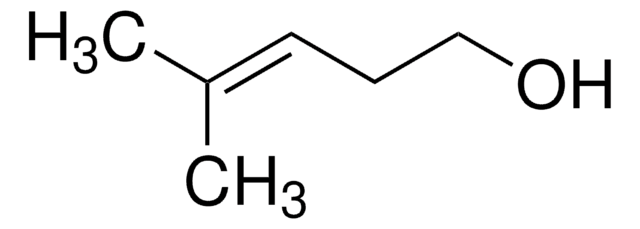
Linear Formula:
(CH3)2C=CHCH2OH
CAS Number:
Molecular Weight:
86.13
Beilstein:
1633479
EC Number:
MDL number:
UNSPSC 코드:
12352100
eCl@ss:
39020334
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
1.4 mmHg ( 20 °C)
Quality Level
분석
99%
양식
liquid
expl. lim.
16.3 %
refractive index
n20/D 1.443 (lit.)
bp
140 °C (lit.)
density
0.848 g/mL at 25 °C (lit.)
작용기
hydroxyl
SMILES string
C\C(C)=C\CO
InChI
1S/C5H10O/c1-5(2)3-4-6/h3,6H,4H2,1-2H3
InChI key
ASUAYTHWZCLXAN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Methyl-2-buten-1-ol reacts with nitrosocarbonyl benzene to yield 5-hydroxy-isoxazolidines. It is commonly used as fragrance ingredient.
애플리케이션
3-Methyl-2-buten-1-ol was used as starting reagent during asymmetric total syntheses of (R)-(+)- and (S)-(-)-umbelactone via Sharpless asymmetric epoxidation reaction.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
124.7 °F - closed cup
Flash Point (°C)
51.5 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Anastasia Zerva et al.
Molecules (Basel, Switzerland), 23(9) (2018-09-22)
Feruloyl esterases (FAEs, E.C. 3.1.1.73) are biotechnologically important enzymes with several applications in ferulic acid production from biomass, but also in synthesis of hydroxycinnamic acid derivatives. The use of such biocatalysts in commercial processes can become feasible by their immobilization
Huawei Liu et al.
Chirality, 18(4), 223-226 (2006-03-08)
The asymmetric total syntheses of (R)-(+)- and (S)-(-)-umbelactone were achieved by using the Sharpless asymmetric epoxidation reaction to generate the stereogenic center and a ring-closing metathesis (RCM) for the formation of the lactone structure. Starting from 3-methyl-2-buten-1-ol, the asymmetric total
Alvaro Acosta-Serrano et al.
Eukaryotic cell, 3(2), 255-263 (2004-04-13)
Concanavalin A (ConA) kills the procyclic (insect) form of Trypanosoma brucei by binding to its major surface glycoprotein, procyclin. We previously isolated a mutant cell line, ConA 1-1, that is less agglutinated and more resistant to ConA killing than are
Yi-Fan Chang et al.
The Journal of organic chemistry, 73(18), 7197-7203 (2008-08-19)
Solid-phase organic synthesis of polyprenols with a traceless sulfone linker is described. The polymer-bound benezenesulfinate is first linked with the "tail" building blocks of isoprenyl chlorides via S-alkylation. With use of dimsyl anion as an appropriate base, the polymer-bound alpha-sulfonyl
Geetu Singh et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 14(12), 792-798 (2007-08-11)
Ethanol extract of Coccinia grandis (L.) Voigt showed significant triglyceride (TG) and cholesterol-lowering effects in dyslipidemic hamster model. Ethanolic extract was fractionated into chloroform, n-butanol and water-soluble fractions and were evaluated. Activity was proved to be concentrated in chloroform-soluble fraction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.