추천 제품
제품명
CDI, reagent grade
Grade
reagent grade
Quality Level
분석
≥90.0% (proton, NMR)
양식
solid
반응 적합성
reaction type: Carbonylations
환경친화적 대안 제품 특성
Designing Safer Chemicals
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
117-122 °C (lit.)
응용 분야
peptide synthesis
환경친화적 대안 카테고리
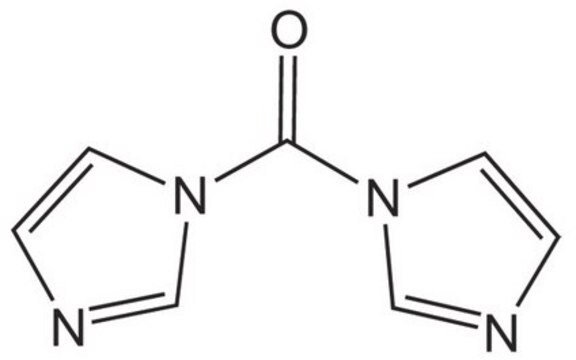
SMILES string
O=C(n1ccnc1)n2ccnc2
InChI
1S/C7H6N4O/c12-7(10-3-1-8-5-10)11-4-2-9-6-11/h1-6H
InChI key
PFKFTWBEEFSNDU-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 115533-1KG | 4061826731888 |
| 115533-250G | |
| 115533-100G | 4061838703323 |
| 115533-10G | 4061838703330 |
| 115533-25G | 4061838703347 |
| 115533-25KG | 4061833431450 |
| 115533-500G | 4061838703354 |
| 115533-5G | 4061838703361 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.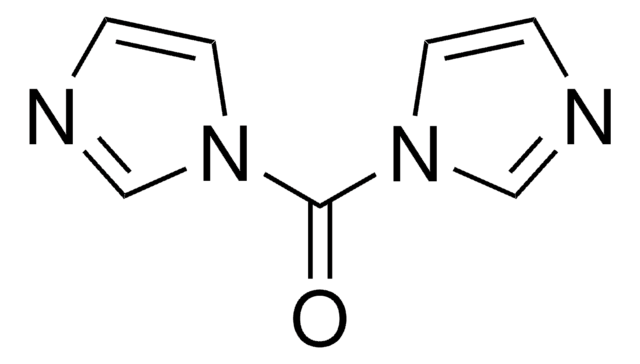
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)