1A00490
USP
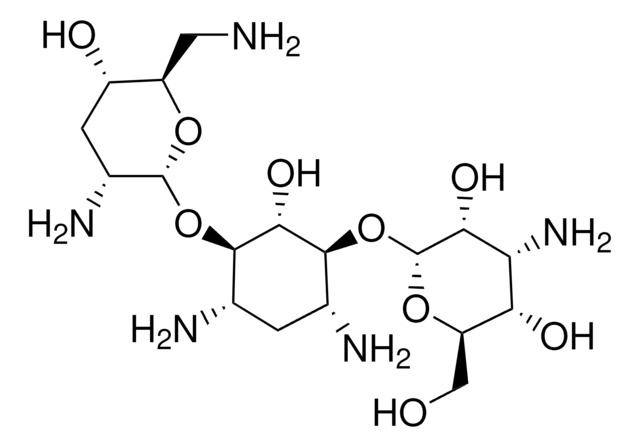
Amoxicilloic Acid Dimers 1 And 2
Pharmaceutical Analytical Impurity (PAI)
別名:
((4S)-2-[1-[[(2R)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino]-2-[[(1R)-2-[[carboxy[(4S)-4-carboxy-5,5-dimethyl-1,3- thiazolidin-2-yl]methyl]amino]-1-(4-hydroxyphenyl)-2-oxoethyl]amino]-2-oxoethyl]-5,5-dimethyl-1,3-thiazolidine-4- carboxylic Acid)
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
グレード
pharmaceutical analytical impurity (PAI)
認証
USP
メーカー/製品名
USP
アプリケーション
pharmaceutical
フォーマット
neat
保管温度
−20°C
詳細
Amoxicilloic Acid Dimers 1 And 2 is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Amoxicillin
Therapeutic Area: Antibiotics.
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Amoxicillin
Therapeutic Area: Antibiotics.
For more information about this PAI, visit here.
アプリケーション
Amoxicilloic Acid Dimers 1 And 2 (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
特徴および利点
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
1A00490-10MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)