おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
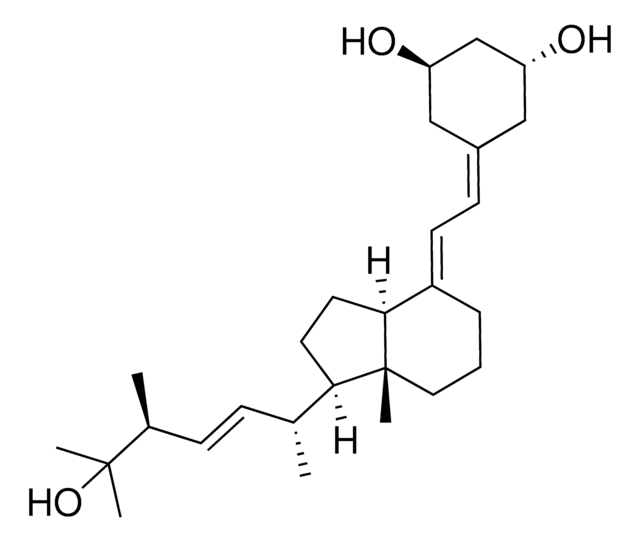
paricalcitol
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
−20°C
SMILES記法
O[C@H]1C[C@@H](CC(=C\C=C2\[C@H]3[C@@]([C@H](CC3)[C@H](C)\C=C\[C@@H](C(O)(C)C)C)(CCC\2)C)C1)O
InChI
1S/C27H44O3/c1-18(8-9-19(2)26(3,4)30)24-12-13-25-21(7-6-14-27(24,25)5)11-10-20-15-22(28)17-23(29)16-20/h8-11,18-19,22-25,28-30H,6-7,12-17H2,1-5H3/b9-8+,21-11+/t18-,19+,22-,23-,24-,25+,27-/m1/s1
InChI Key
BPKAHTKRCLCHEA-UBFJEZKGSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Paricalcitol USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
保管分類コード
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Darko Duplancic et al.
Clinical interventions in aging, 8, 149-156 (2013-02-23)
The ubiquitous distribution of vitamin D receptors in the human body is responsible for the pleiotropic effects of vitamin D-receptor activation. We discuss the possible beneficial effects of a selective activator of vitamin D receptor, paricalcitol, on the cardiovascular system
Mario Cozzolino et al.
Current vascular pharmacology, 6(2), 148-153 (2008-04-09)
Hemodialysis (HD) patients are commonly affected by secondary hyperparathyroidism (SHPT), in which 3 well-known factors are usually involved: hypocalcemia, hyperphosphatemia and calcitriol deficiency. Classically, high parathyroid hormone (PTH) levels cause bone-associated diseases, such as osteitis fibrosa and renal osteodystrophy, but
H Amer et al.
American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 13(6), 1576-1585 (2013-04-23)
Postkidney transplant hyperparathyroidism is a significant problem. Vitamin D receptor agonists are known to suppress parathyroid hormone (PTH) secretion. We examined the effect of oral paricalcitol on posttransplant secondary hyperparathyroidism by conducting an open label randomized trial in which 100
Dean M Robinson et al.
Drugs, 65(4), 559-576 (2005-03-01)
Paricalcitol (Zemplar) is a synthetic vitamin D(2) analogue that inhibits the secretion of parathyroid hormone (PTH) through binding to the vitamin D receptor. It is approved in the US and in most European nations for intravenous use in the prevention
Mario Cozzolino et al.
Contributions to nephrology, 171, 161-165 (2011-06-01)
Cardiovascular (CV) morbidity and mortality are significantly higher in patients with chronic kidney disease (CKD). Mineral metabolism disorders, such as hyperphosphatemia, hypocalcemia, and vitamin D deficiency, have been deeply associated not only with bone disease, but also with vascular calcification
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)