すべての画像(1)
About This Item
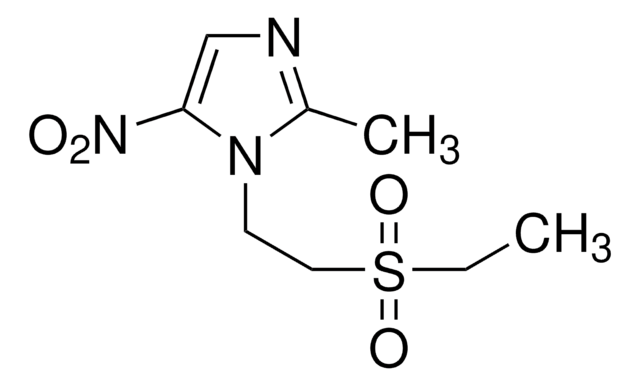
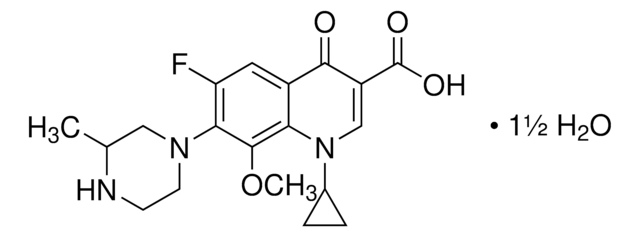
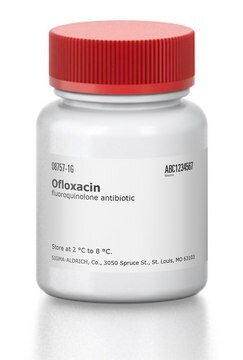
実験式(ヒル表記法):
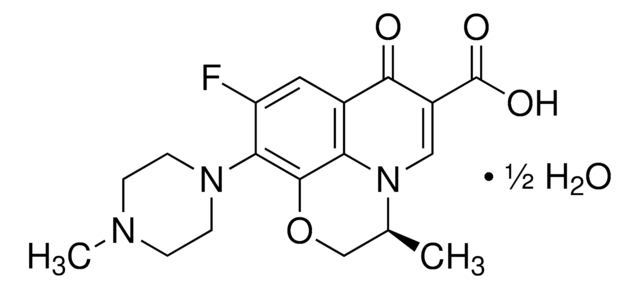
C18H20FN3O4
CAS番号:
分子量:
361.37
MDL番号:
UNSPSCコード:
41116107
PubChem Substance ID:
NACRES:
NA.24
おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
ofloxacin, ofloxacin
メーカー/製品名
USP
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
CC1COc2c(N3CCN(C)CC3)c(F)cc4C(=O)C(=CN1c24)C(O)=O
InChI
1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)
InChI Key
GSDSWSVVBLHKDQ-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Ofloxacin USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
Also, for use with USP monographs such as:
Also, for use with USP monographs such as:
- Ofloxacin Tablets
- Ofloxacin Ophthalmic Solution
- Levofloxacin
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 2
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
1478108-200MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
J P Monk et al.
Drugs, 33(4), 346-391 (1987-04-01)
Ofloxacin is one of a new generation of fluorinated quinolones structurally related to nalidixic acid. It is an orally administered broad spectrum antibacterial drug active against most Gram-negative bacteria, many Gram-positive bacteria and some anaerobes. Ciprofloxacin is the only other
Mahesh D Chavanpatil et al.
International journal of pharmaceutics, 316(1-2), 86-92 (2006-03-29)
Oral sustained release gastroretentive dosage forms offer many advantages for drugs having absorption from upper gastrointestinal tract and improve the bioavailability of medications that are characterized by a narrow absorption window. A new gastroretentive sustained release delivery system was developed
Shu-Hwa Hsiao et al.
The Annals of pharmacotherapy, 39(1), 146-149 (2004-11-25)
To report a case of ofloxacin/levofloxacin-induced rhabdomyolysis and to compare other reported cases from the literature. A 19-year-old male patient developed ofloxacin/levofloxacin-induced rhabdomyolysis during admission for periorbital cellulitis. Symptoms of myalgia, weakness, and swelling of the arms developed after 3
S M Traeger et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 21(6), 1504-1506 (1995-12-01)
We describe four patients who had seizures while receiving ofloxacin; no other causes were evident. Common factors among all patients included advanced age and use of a high-dose regimen. The renal insufficiency of three patients and the timing of the
G A Gates
The Pediatric infectious disease journal, 20(1), 104-107 (2001-02-15)
To assess the safety of topical agents in the middle ear, animal studies were reviewed. Compared with aminoglycoside-containing preparations, which caused significant loss of hair cells in the basal turn of the cochlea, ofloxacin caused no loss of hair cells
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)


![(S)-(-)-9,10-ジフルオロ-2,3-ジヒドロ-3-メチル-7-オキソ-7H-ピリド-[1,2,3-de]-1,4-ベンゾオキサジン-6-カルボン酸 United States Pharmacopeia (USP) Reference Standard](/deepweb/assets/sigmaaldrich/product/structures/177/773/83bb0da1-8f11-4fd8-8f74-d32b5f43f2ab/640/83bb0da1-8f11-4fd8-8f74-d32b5f43f2ab.png)