SML1669
NCX 4016
≥98% (HPLC)
別名:
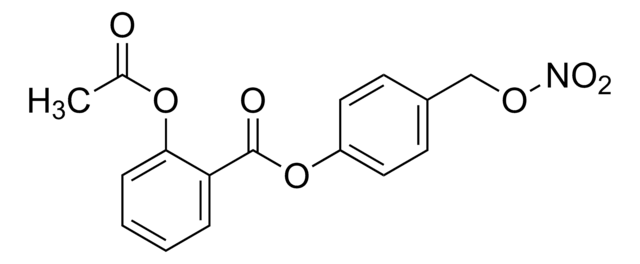
2-(Acetyloxy)-benzoic acid 3-[(nitrooxy)methyl]phenyl ester, 2-Acetoxybenzoic acid 3-nitrooxymethylphenyl ester, 3-(Nitroxymethyl)phenyl 2-acetoxybenzoate, NCX-4016, Nitroaspirin
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C16H13NO7
CAS番号:
分子量:
331.28
MDL番号:
UNSPSCコード:
51111800
PubChem Substance ID:
NACRES:
NA.77
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
powder
色
white to beige
溶解性
DMSO: 25 mg/mL, clear
保管温度
−20°C
SMILES記法
CC(OC1=CC=CC=C1C(OC2=CC(CO[N+]([O-])=O)=CC=C2)=O)=O
InChI
1S/C16H13NO7/c1-11(18)23-15-8-3-2-7-14(15)16(19)24-13-6-4-5-12(9-13)10-22-17(20)21/h2-9H,10H2,1H3
InChI Key
IOJUJUOXKXMJNF-UHFFFAOYSA-N
生物化学的/生理学的作用
NCX 4016 (nitroaspirin) is nitroderivative of aspirin that combines cyclooxygenase inhibitor with an NO donor. NCX 4016 improves postischemic ventricular dysfunction and to reduce myocardial infarct size in rabbit.
NCX-4016 can prevent the progression of cisplatin-resistant human ovarian cancer xenografts in mice. It can also regulate the expression of B-cell lymphoma 2 (Bcl-2) proteins in ovarian cancer cells. Hence, NCX-4016 is considered a promising therapeutic option against carcinoma.
Nitroderivative of aspirin that combines cyclooxygenase inhibitor with an NO donor
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
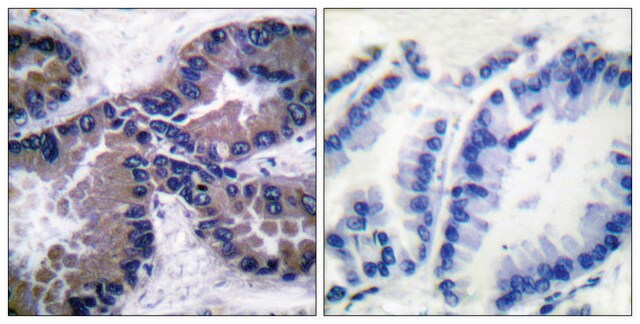
Karuppaiyah Selvendiran et al.
Cell cycle (Georgetown, Tex.), 7(1), 81-88 (2008-01-17)
We have previously reported the inhibitory effect of NCX-4016, a nitro derivative of aspirin, on the proliferation of cisplatin-resistant human ovarian cancer cells, in vitro (Bratasz et al., Proc Natl Acad Sci USA 2006; 103:3914-9). In this report we present
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)