おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
powder
保管条件
desiccated
色
light yellow to light brown
溶解性
DMSO: >2 mg/mL (warmed)
保管温度
2-8°C
SMILES記法
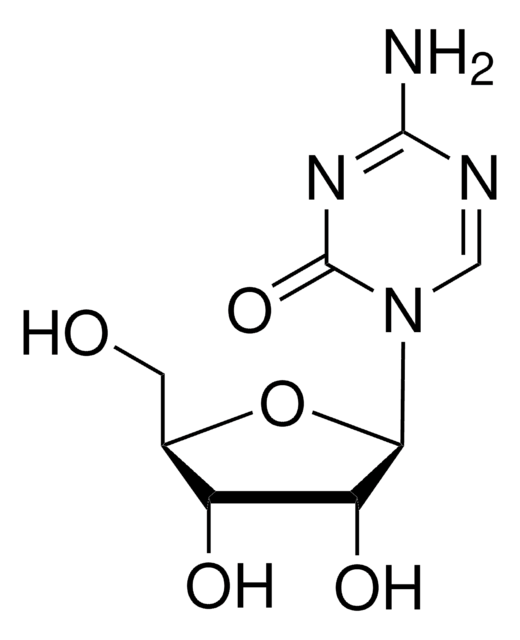
COc1ccc(cc1)N2N=C3C(=CNc4ccccc34)C2=O
InChI
1S/C17H13N3O2/c1-22-12-8-6-11(7-9-12)20-17(21)14-10-18-15-5-3-2-4-13(15)16(14)19-20/h2-10,18H,1H3
InChI Key
KSKRJZMRHSNRBX-UHFFFAOYSA-N
生物化学的/生理学的作用
CGS 9895 is a GABA antagonist that acts via the benzodiazepine binding site of ag containing GABA receptors. CGS 9895 is the only known compound that can specifically enhance GABA-induced currents in ab subunit containing receptors, and acts at the extracellular a1+b3- subunit interface.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0376-5MG:
SML0376-VAR:
SML0376-BULK:
SML0376-IP:
SML0376-25MG:
最新バージョンのいずれかを選択してください:
Behavioral effects of benzodiazepine antagonists in chlordiazepoxide tolerant and non-tolerant rats.
K Takada et al.
Life sciences, 44(4), 289-299 (1989-01-01)
Rats were trained to respond under 3-min fixed-interval schedules of food presentation, and effects of the benzodiazepine-receptor ligands, flumazenil, 2-(4-methoxy-phenyl)-pyrazolo[4,3-c]quinolin-3(5H)-one (CGS 9895), 3-carbo-t-butoxy-beta-carboline (beta-CCtB), and beta-carboline-3-carboxylic acid ethyl ester (beta-CCE) were assessed before and after the induction of tolerance to
N J Katzman et al.
The Journal of pharmacology and experimental therapeutics, 235(3), 589-595 (1985-12-01)
CGS 9895, a pyrazoloquinolone benzodiazepine receptor ligand, was administered alone and concomitantly with diazepam in order to assess its agonist and diazepam-antagonist properties on various behaviors in rodents. In mice, CGS 9895 neither potentiated nor blocked the convulsant effects of
H E Shannon et al.
European journal of pharmacology, 92(1-2), 155-157 (1983-08-19)
The diazepam-like agonist and diazepam antagonist properties of the pyrazoloquinoline benzodiazepine receptor ligands CGS8216, CGS9895 and CGS9896 were evaluated in rats trained to discriminate between saline and 1.0 mg/kg of diazepam in a two-choice, discrete-trial procedure. None of the three
H E Shannon et al.
Pharmacology, biochemistry, and behavior, 23(2), 317-323 (1985-08-01)
The effects of diazepam and the pyrazoloquinoline benzodiazepine receptor ligands CGS8216, CGS9896, and CGS9895 on schedule-controlled responding were studied in dogs. Responding was maintained under a multiple fixed-interval (FI) 5-min fixed-ratio (FR) 30 response schedule of food presentation. Diazepam (PO)
L T Schove et al.
Medicinal chemistry research : an international journal for rapid communications on design and mechanisms of action of biologically active agents, 4(5), 307-314 (1994-01-01)
Four pyrazoloquninolinone compounds, variations of known high affinity ligands for the GABA A/Benzodiazepine receptors (BDZRs), were synthesized and their affinities for BDZRs in cerebellum and spinal cord measured and compared to the parent compounds, CGS 8216, CGS 9895, and CGS
資料
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)