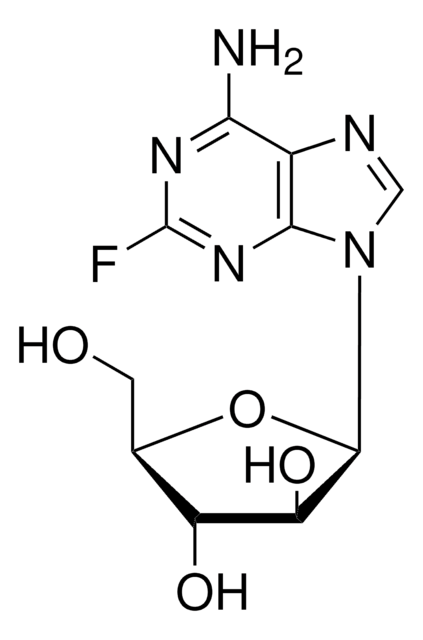
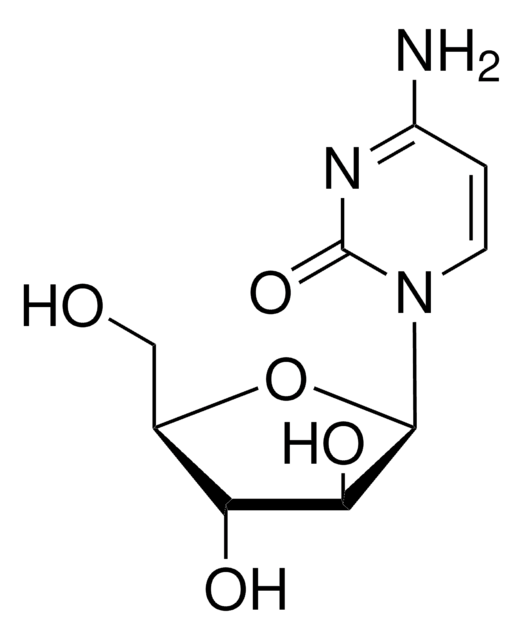
おすすめの製品
フォーム
powder
品質水準
色
white
溶解性
DMSO: soluble
抗生物質活性スペクトル
neoplastics
作用機序
DNA synthesis | interferes
保管温度
−20°C
SMILES記法
Fc1nc2[n](cnc2c(n1)[N+H3])C3OC(C(C3O)O)CO[P](=O)([O-])O
InChI
1S/C10H13FN5O7P/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,14,15)(H2,19,20,21)
InChI Key
GIUYCYHIANZCFB-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
Fludarabine is a purine analog included in the category of DNA-damaging drugs with well-known efficacy in B-cell chronic lymphocytic leukemia (B-CLL).
アプリケーション
- Characterization of Chemical Interactions between Clinical Drugs and the Oral Bacterium, Corynebacterium matruchotii, via Bioactivity-HiTES.: This study explores the interactions of clinical drugs like Fludarabine phosphate with Corynebacterium matruchotii, highlighting potential impacts on oral microbiota and implications for drug efficacy and safety (Lee DY et al., 2024).
- Cocktail of lipophilic and hydrophilic chemotherapeutics in high-load core@shell nanocarriers to treat pancreatic tumours.: Investigates the efficacy of a combination of Fludarabine phosphate with other chemotherapeutics delivered via nanocarriers, aiming to enhance treatment outcomes for pancreatic cancer by improving drug delivery to the tumor site (Rudolph D et al., 2024).
- Macrophage neogenin deficiency exacerbates myocardial remodeling and inflammation after acute myocardial infarction through JAK1-STAT1 signaling.: This research demonstrates the role of Fludarabine phosphate in modulating inflammation and cardiac repair post-myocardial infarction, offering insights into its potential therapeutic benefits beyond oncology (Zhang J et al., 2023).
- SLC25A51 promotes tumor growth through sustaining mitochondria acetylation homeostasis and proline biogenesis.: Discusses the cellular mechanisms by which Fludarabine phosphate may influence metabolic pathways in cancer cells, highlighting its potential to disrupt tumor metabolism and promote cancer cell death (Li Y et al., 2023).
- CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome.: Reviews the use of Fludarabine phosphate in preconditioning regimens for CAR T-cell therapy, emphasizing its role in enhancing the efficacy of immunotherapy in treating autoimmune disorders (Pecher AC et al., 2023).
生物化学的/生理学的作用
Fludarabine represses DNA replication and suppresses the nucleotide metabolism by inhibiting the enzyme ribonucleotide reductase.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Muta. 2 - Repr. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
F9813-BULK:
F9813-25MG-PW:
F9813-VAR:
F9813-5MG-PW:
F9813-5MG:
F9813-25MG:
この製品を見ている人はこちらもチェック
Francesca Ricci et al.
Therapeutics and clinical risk management, 5(1), 187-207 (2009-05-14)
Fludarabine (FAMP) is the most effective and most extensively studied purine analog in indolent B-cell malignancies. Its use is indicated for first-and second-line treatment of B-cell chronic lymphocytic leukemia (B-CLL). FAMP as a single agent has produced superior response rates
Andrea Celeghin et al.
Cell death & disease, 7(12), e2562-e2562 (2016-12-30)
Besides its canonical role in stabilizing telomeres, telomerase reverse transcriptase (TERT) may promote tumorigenesis through extra-telomeric functions. The possible therapeutic effects of BIBR1532 (BIBR), a powerful TERT inhibitor, have been evaluated in different cellular backgrounds, but no data are currently
Míriam Molina-Arcas et al.
Blood, 101(6), 2328-2334 (2002-11-02)
Nucleoside derivatives are currently used in the treatment of hematologic malignancies. Although intracellular events involved in the pharmacologic action of these compounds have been extensively studied, the role of plasma membrane transporters in nucleoside-derived drug bioavailability and action in leukemia
W Plunkett et al.
Seminars in oncology, 17(5 Suppl 8), 3-17 (1990-10-01)
Fludara I.V. (fludarabine phosphate) (9-beta-D-arabinosyl-2-fluoroadenine, F-ara-A) is an adenine nucleoside analogue resistant to adenosine deaminase that shows promising therapeutic activity in the clinical treatment of lymphocytic hematologic malignancies. F-ara-A is transported into cells, where it is converted to its 5'-triphosphate
H M Kantarjian et al.
Seminars in oncology, 17(5 Suppl 8), 66-70 (1990-10-01)
The promising results obtained with Fludara I.V. (fludarabine phosphate) treatment in the common indolent B-cell neoplasms have led to their further evaluation in other unusual B-cell malignancies, in Hodgkin's disease, and in T-cell diseases. A significant response rate has been
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)