おすすめの製品
品質水準
アッセイ
≥99%
フォーム
powder
溶解性
warm ethanol: 10 mg/mL, clear to slightly hazy, colorless to faintly yellow
保管温度
2-8°C
SMILES記法
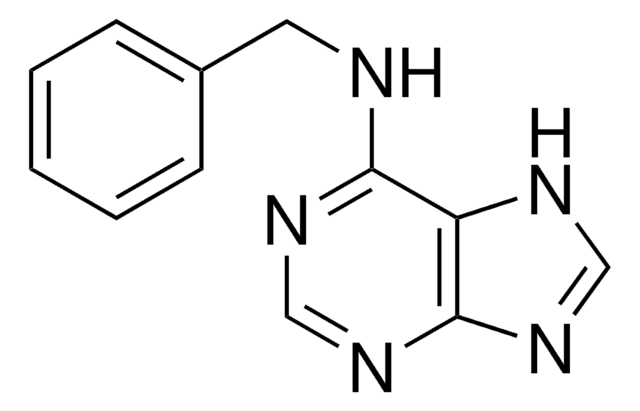
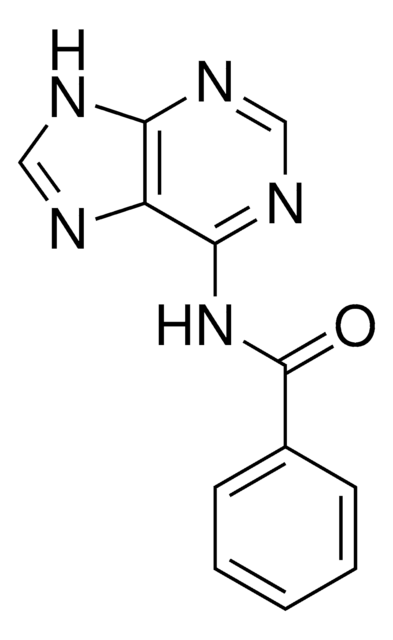
O=C(Nc1ncnc2nc[nH]c12)c3ccccc3
InChI
1S/C12H9N5O/c18-12(8-4-2-1-3-5-8)17-11-9-10(14-6-13-9)15-7-16-11/h1-7H,(H2,13,14,15,16,17,18)
InChI Key
QQJXZVKXNSFHRI-UHFFFAOYSA-N
関連するカテゴリー
アプリケーション
N6-Benzoyladenine is used in the organic synthesis of adenine derivative molecules such as bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine and to produce oxy-peptide nucleic acids.
生物化学的/生理学的作用
N6-Benzoyladenine comprises of adenine moiety and is a potent inhibitor of bromodomain-containing protein 4 (BRD4). N6-Benzoyladenine modulates tumor necrosis factor α (TNF-α) levels. It elicits cytotoxicity in liver and ileum cancer cells and may serve as a potential candidate agent for cancer chemotherapy.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Repr. 2 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
B5258-VAR:
B5258-1G:
B5258-BULK:
B5258-25G:
B5258-5G:
Structure-Activity Relationship Study of N6-Benzoyladenine-Type BRD4 Inhibitors and Their Effects on Cell Differentiation and TNF-alpha Production
Amemiya S, et al.
Chemical & Pharmaceutical Bulletin, 64(9), 1378-1383 (2016)
M Kuwahara et al.
Nucleic acids symposium series, 42(42), 31-32 (2000-04-26)
Syntheses of N-Fmoc delta-amino acids with an ether linkage in the main chain and six different nucleobases on the side chain, Fmoc-NH-C*H(CH2-CH2-B)-CH2-O-CH2-COOH (B = N6-benzoyladenine, thymine, uracil, N-benzoylcytosine, guanine, and N2-isobutyrylguanine) are described. The delta-amino acids were prepared through 8-12
A E Håkansson et al.
Bioorganic & medicinal chemistry letters, 11(7), 935-938 (2001-04-11)
Synthesis of a 9-mer alpha-L-LNA (alpha-L-ribo configured locked nucleic acid) containing three 9-(2-O,4-C-methylene-alpha-L-ribofuranosyl)adenine nucleotide monomer(s) has been accomplished. The work involved synthesis of the bicyclic adenine nucleoside via a condensation reaction between L-threo-pentofuranose derivative 1 and 6-N-benzoyladenine followed by C2'-epimerization.
Discovery and structure-activity relationship studies of N6-benzoyladenine derivatives as novel BRD4 inhibitors
Noguchi-Yachide T, et al.
Bioorganic & Medicinal Chemistry, 23(5), 953-959 (2015)
Synthesis and cytotoxicity of cyanoborane adducts of n6-benzoyladenine and 6-triphenylphosphonylpurine
Scarlett TC, et al.
Metal-based drugs, 9(1-2), 19-32 (2002)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)