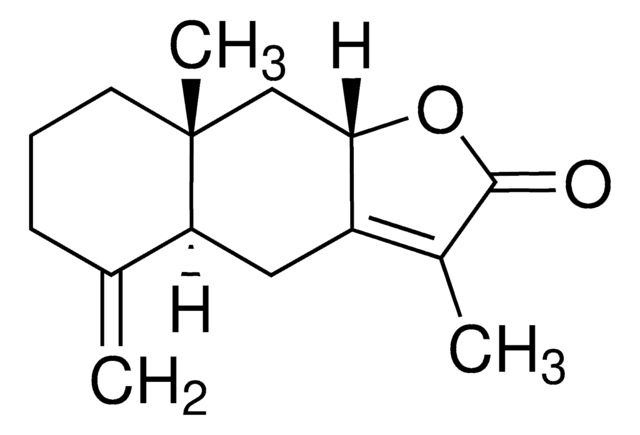
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
powder or crystals
溶解性
methanol: 1 mg/mL, clear, colorless
アプリケーション
metabolomics
vitamins, nutraceuticals, and natural products
保管温度
2-8°C
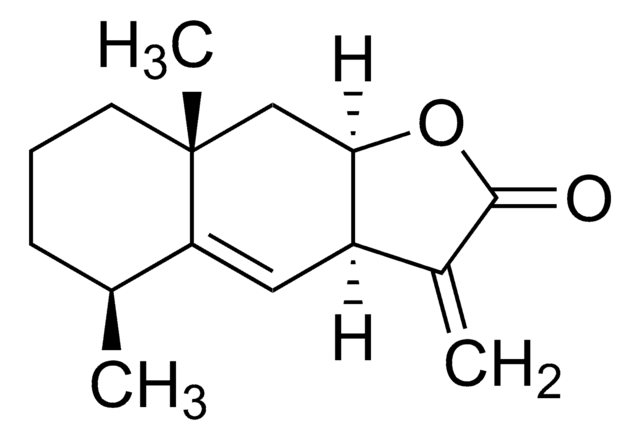
SMILES記法
C=C1CCC[C@@](C=C2O3)(C)[C@@]1([H])CC2=C(C)C3=O
InChI
1S/C15H18O2/c1-9-5-4-6-15(3)8-13-11(7-12(9)15)10(2)14(16)17-13/h8,12H,1,4-7H2,2-3H3/t12-,15+/m0/s1
InChI Key
ZTVSGQPHMUYCRS-SWLSCSKDSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Atractylenolide I is one of the active ingredients of Rhizoma Atractylodes macrocephala which is obtained from the dried root and stem of Atractylodes Macrocephala Koidz. It is a sesquiterpene compound of hydrophobic nature.
アプリケーション
Atractylenolide I is a phytochemical that may be used to study its anti-inflammatory and anti-angiogenesis activities. Atractylenolide I may be used and studied as a postitive modulator of GABA-induced chloride currents I(GABA) and as an inhibitor of aromatases. Atractylenolide I may be used as a reference material in assays to detect its presence in plant root extracts and biological milieu such as plasma.
生物化学的/生理学的作用
Atractylenolide I has been reported to have an anti-inflammatory activity due to its inhibitory effect on tumor necrosis factor-α (TNF-α) and the production of nitric oxide (NO). It also shows anti-cancer nature, thereby being used in the management of gastric cancer cachexia symptoms. Additionally, it has anti-angiogenic, pro-oxidative and cytotoxic characteristics.
Phytochemical from Traditional Chinese Medicine herbal preparations. Atractylenolide I is an anti-inflammatory that is reported to inhibit angiogenesis.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
339.6 °F
引火点(℃)
170.87 °C
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
A2737-VAR:
A2737-10MG:
A2737-50MG:
A2737-BULK:
最新バージョンのいずれかを選択してください:
試験成績書(COA)
Lot/Batch Number
Judith Singhuber et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 19(3-4), 334-340 (2011-11-29)
Several Chinese herbal medicines (CHMs) are used in the treatment of insomnia, restlessness, or anxiety. However, mechanisms underlying this effect and scientific proof for their traditional use is scarce. In the present study CHMs were screened for their ability to
Hyun Young Park et al.
Planta medica, 77(13), 1528-1530 (2011-02-25)
The roots of Cyathula officinalis Kuan are widely used in Chinese medicine for the treatment of inflammatory disorders. Here, the ability of C. officinalis Kuan to downregulate matrix metalloproteinase (MMP)-13 was examined since MMP-13 is an important enzyme for the
Jian-Ming Huang et al.
Scientific reports, 4, 3840-3840 (2014-01-24)
Paclitaxel, a known TLR4 ligand, leads to activation of TLR4/MyD88-dependent pathway that mediates chemoresistance and tumor progression in epithelial ovarian carcinoma (EOC). Atractylenolide-I (AO-I), a novel TLR4-antagonizing agent, inhibits TLR4 signaling by interfering with the binding of LPS or paclitaxel
Yujuan Li et al.
Journal of pharmaceutical and biomedical analysis, 58, 172-176 (2011-10-22)
A new high-performance liquid chromatography/tandem mass spectrometry (LC-MS/MS) was developed for quantitative analysis of atractylenolide I in rat plasma using buspirone as internal standard (I.S.). Rat plasma samples were deproteined with methanol and acetonitrile (1:1, v/v). Atractylenolide I and I.S.
Hai Jiang et al.
Molecules (Basel, Switzerland), 16(4), 3146-3151 (2011-04-16)
Ten compounds were isolated from the dichloromethane extract of Atractylodes macrocephala and their aromatase inhibiting activities were tested using an in vitro fluorescent-based aromatase assay. The results indicated that atractylenolide I, atractylenolide II and atractylenolide III had inhibition ratios of
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)