おすすめの製品
アッセイ
≥90% (HPLC)
メーカー/製品名
ATTO-TEC GmbH
λ
in methanol
UV吸収
λ: 429-435 nm Amax
適合性
suitable for fluorescence
保管温度
−20°C
詳細
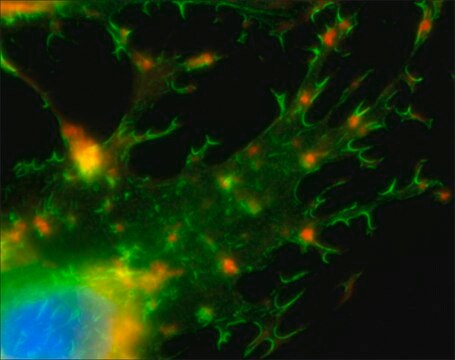
Atto 425 Phalloidin is a novel fluorescent label with a coumarin structure. The dye is intended for application in the area of life science, e.g. labeling of DNA, RNA or proteins. Characteristic features of the label are strong absorption, high fluorescence quantum yield, large Stokes-shift, good photostability, and low molecular weight.Phalloidin is a fungal toxin isolated from the poisonous mushroom Amanita phalloides. Its toxicity is attributed to the ability to bind F actin in liver and muscle cells. As a result of binding phalloidin, actin filaments become strongly stabilized. Phalloidin has been found to bind only to polymeric and oligomeric forms of actin, and not to monomeric actin. The dissociation constant of the actin-phalloidin complex has been determined to be on the order of 3 x 10–8. Phalloidin differs from amanitin in rapidity of action; at high dose levels, death of mice or rats occurs within 1 or 2 hours.
アプリケーション
Fluorescent conjugates of phalloidin, rhodamine-phalloidin staining reagents, such as Phalloidin–Atto 425 are used to label actin filaments for histological applications. Some structural features of phalloidin are required for the binding to actin. However, the side chain of amino acid 7 (g-dihydroxyleucine) is accessible for chemical modifications without appreciable loss of affinity for actin.
法的情報
本製品は研究専用です。商業目的で使用する場合は、ライセンス取得について知的所有権(IP)保持者(ATTO-TEC GmbH、ドイツ)にお問い合わせください。
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
66939-10NMOL:
Louise R Page
Evolution & development, 4(3), 212-222 (2002-06-11)
Results of this study on two species of vetigastropods contradict the long-standing hypothesis, originally proposed by Garstang (1929), that the larval retractor muscles power the morphogenetic movement of ontogenetic torsion in all basal gastropods. In the trochid Calliostoma ligatum and
Rosangela Invernizzi et al.
Oncology, 75(3-4), 237-244 (2008-10-16)
Pegfilgrastim is a covalent conjugate of filgrastim and polyethylene glycol that has proved to be effective in supporting myelopoiesis during chemotherapy. Since very limited information is available on the biological effects of pegfilgrastim on neutrophils exposed to chemotherapy, we analyzed
Takahiro Shimizu et al.
Cell calcium, 45(3), 226-232 (2008-12-03)
We demonstrate here that the transient receptor potential melastatin subfamily channel, TRPM4, controls migration of bone marrow-derived mast cells (BMMCs), triggered by dinitrophenylated human serum albumin (DNP-HSA) or stem cell factor (SCF). Wild-type BMMCs migrate after stimulation with DNP-HSA or
Stimulated emission depletion-based raster image correlation spectroscopy reveals biomolecular dynamics in live cells.
Hedde P.N.; et al.
Nature Communications, 4, 2093-2093 (2013)
SNARE Function Is Not Involved in Early Endosome Docking.
Geumann, U.; et al.
Molecular Biology of the Cell, 19(12), 5327-5337 (2008)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)