Y0000902
ラセカドトリル
European Pharmacopoeia (EP) Reference Standard
別名:
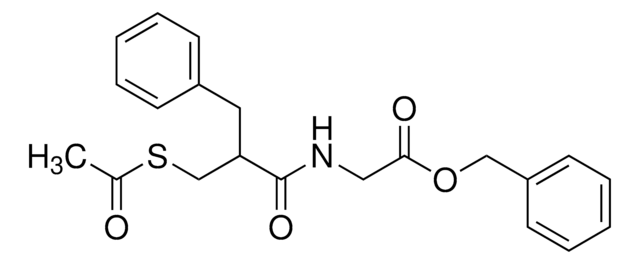
(RS)-ベンジル N-[3-(アセチルチオ)-2-ベンジルプロパノイル]グリシナート, (±)-アセトルファン, Cadotril, Redotil, Tiorfan, Zedott, アセトルファン
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
C21H23NO4S
CAS番号:
分子量:
385.48
MDL番号:
UNSPSCコード:
41116107
PubChem Substance ID:
NACRES:
NA.24
おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
racecadotril
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
CC(=O)SCC(Cc1ccccc1)C(=O)NCC(=O)OCc2ccccc2
InChI
1S/C21H23NO4S/c1-16(23)27-15-19(12-17-8-4-2-5-9-17)21(25)22-13-20(24)26-14-18-10-6-3-7-11-18/h2-11,19H,12-15H2,1H3,(H,22,25)
InChI Key
ODUOJXZPIYUATO-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Racecadotril for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生物化学的/生理学的作用
ラセカドトリルは天然のエンドペプチダーゼインヒビターで、末梢作動性エンケファリナーゼインヒビターとして働く止痢薬です。
天然のエンドペプチダーゼインヒビター、止痢薬
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Aquatic Acute 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
J I Emparanza Knörr et al.
Anales de pediatria (Barcelona, Spain : 2003), 69(5), 432-438 (2009-01-09)
To estimate, through a systematic review of the literature, the efficacy of racecadotril in the treatment of acute diarrhoea. Randomised trials carried out in children comparing racecadotril with placebo in terms of diarrhoea recovery, stools output and adverse effects were
Ramón Tormo et al.
Acta paediatrica (Oslo, Norway : 1992), 97(8), 1008-1015 (2008-05-09)
In developing countries acute infectious diarrhoea remains one of the leading causes of death among young children, especially those under 1 year of age. In contrast, in industrialized nations the death rate is very low, although the disease is an
J M Lecomte
International journal of antimicrobial agents, 14(1), 81-87 (2000-03-16)
Since preclinical studies had indicated the potential efficacy and tolerability of racecadotril for the treatment of diarrhoea in man, a series of studies was carried out to assess the clinical effects of racecadotril. These studies were also designed to evaluate
S Huighebaert et al.
Digestive diseases and sciences, 48(2), 239-250 (2003-03-20)
Racecadotril is an enkephalinase inhibitor, presented as a purely antisecretory agent with advantages over the opiate-receptor agonist loperamide in the treatment of diarrhea. A critical review of the literature and the models used was performed. Although pretreatment with high doses
H Szajewska et al.
Alimentary pharmacology & therapeutics, 26(6), 807-813 (2007-09-05)
Racecadotril (acetorphan) is an antisecretory drug that exerts its antidiarrhoeal effects by inhibiting intestinal enkephalinase. To summarize studies testing the efficacy and safety of racecadotril for treating children with acute gastroenteritis. Reports were gathered by searching electronic databases MEDLINE, EMBASE
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)