おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
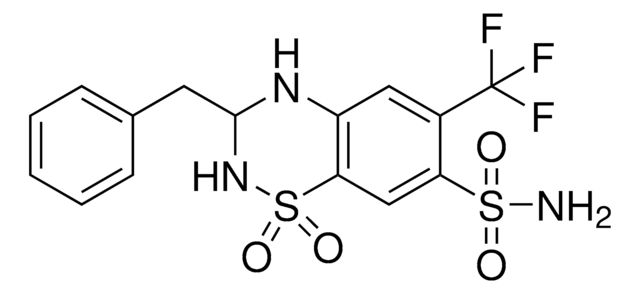
clopamide
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
C[C@@H]1CCC[C@H](C)N1NC(=O)c2ccc(Cl)c(c2)S(N)(=O)=O
InChI
1S/C14H20ClN3O3S/c1-9-4-3-5-10(2)18(9)17-14(19)11-6-7-12(15)13(8-11)22(16,20)21/h6-10H,3-5H2,1-2H3,(H,17,19)(H2,16,20,21)/t9-,10+
InChI Key
LBXHRAWDUMTPSE-AOOOYVTPSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Clopamide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Resp. Sens. 1 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
Y0000833-1EA:
Y0000833:
最新バージョンのいずれかを選択してください:
Validated liquid chromatography?tandem mass spectrometry method for simultaneous determination of clopamide, reserpine and dihydroergotoxine: Application to pharmacokinetics in human plasma.
El-Din M M S, et al.
Journal of Pharmaceutical and Biomedical Analysis, 125, 236-244 (2016)
High-performance liquid chromatographic determination of xipamide and clopamide in pharmaceuticals.
Sane R T, et al.
Journal of Chromatography A, 356, 468-472 (1986)
The Medical journal of Australia, 150(11), 646-646 (1989-06-05)
In an open study that was conducted in general practice, 22 patients with previously-untreated mild hypertension received an average daily dose of 11.7 mg of pindolol over a 50-week study period. The total cholesterol, high-density lipoprotein fraction and plasma triglyceride
Lucie Nováková et al.
Analytica chimica acta, 853, 647-659 (2014-12-04)
The potential and applicability of UHPSFC-MS/MS for anti-doping screening in urine samples were tested for the first time. For this purpose, a group of 110 doping agents with diverse physicochemical properties was analyzed using two separation techniques, namely UHPLC-MS/MS and
Lucie Nováková et al.
Analytica chimica acta, 853, 637-646 (2014-12-04)
The conditions for the analysis of selected doping substances by UHPSFC-MS/MS were optimized to ensure suitable peak shapes and maximized MS responses. A representative mixture of 31 acidic and basic doping agents was analyzed, in both ESI+ and ESI- modes.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)