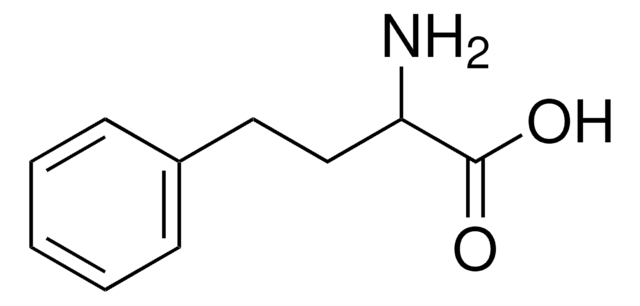
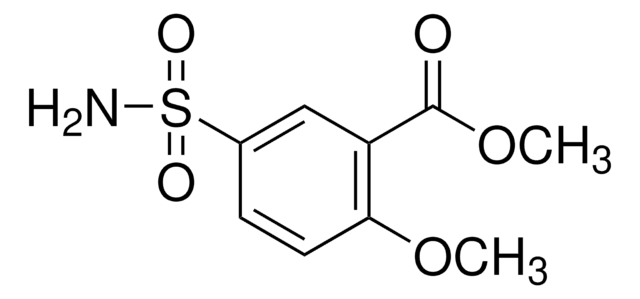
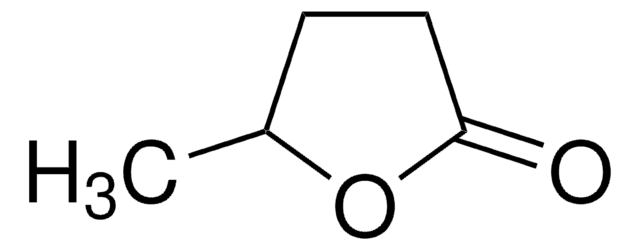
おすすめの製品
詳細
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
アナリシスノート
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAB9790 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
シグナルワード
Warning
危険有害性の分類
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Muta. 2 - Repr. 2 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)