おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to USP 1269061
APIファミリー
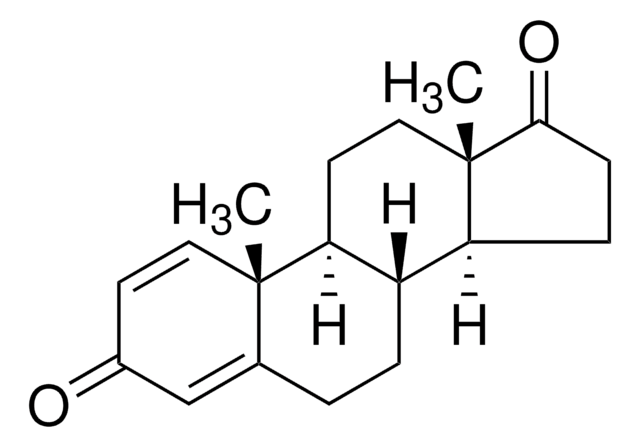
exemestane
CofA
current certificate can be downloaded
包装
pkg of 30 mg
アプリケーション
pharmaceutical
フォーマット
neat
保管温度
2-8°C
SMILES記法
O=C1[C@@]2([C@H]([C@H]3[C@@H]([C@]4(CCC(=O)C=C4C(=C)C3)C)CC2)CC1)C
InChI
1S/C20H26O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h11,14-16H,1,4-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1
InChI Key
KQRGETZTRARSMA-DAELLWKTSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
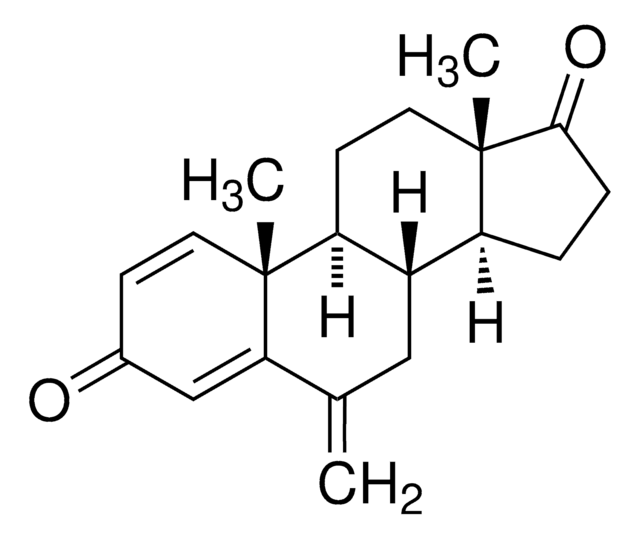
Exemestane Related Compound A is an impurity of the steroidal anticancer drug, exemestane. Exemestane belongs to the class of antiestrogens known as aromatase inhibitors. It is commonly used for the treatment of breast cancer.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
アプリケーション
Exemestane may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by chromatography.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
アナリシスノート
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAB0339 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
関連製品
製品番号
詳細
価格
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1894-30MG-PW:
PHR1894-30MG:
最新バージョンのいずれかを選択してください:
Development and validation of a stability indicating LC method for the assay and related substances determination of Exemestane, an aromatase inhibitor
Kumar RS, et al.
Journal of Pharmaceutical and Biomedical Analysis, 50(5), 746-752 (2009)
Analytical method validation for HPLC assay of oral anticancer drug exemestane
Yavuz B
FABAD Journal of Pharmaceutical Sciences, 32(1), 15-15 (2007)
Exemestane: a review of its clinical efficacy and safety
L?nning PE
Breast (Edinburgh, Scotland), 10(3), 198-208 (2001)
A novel validated stability-indicating RP-HPLC method for the determination of Exemestane (steroidal aromatase inhibitor)
Mukthinuthalapati MA and Bukkapatnam V
Journal of Bioequivalence & Bioavailability, 7(6), 288-288 (2015)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)

![Methyl5-[2-[benzyl(tert-butyl)amino]acetyl]-2-hydroxybenzoate hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material](/deepweb/assets/sigmaaldrich/product/images/192/011/45a62463-4dd5-4639-8d62-60c6e919308a/640/45a62463-4dd5-4639-8d62-60c6e919308a.jpg)
