おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. C0465000
APIファミリー
carbimazole
CofA
current certificate can be downloaded
包装
pkg of 1 g
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
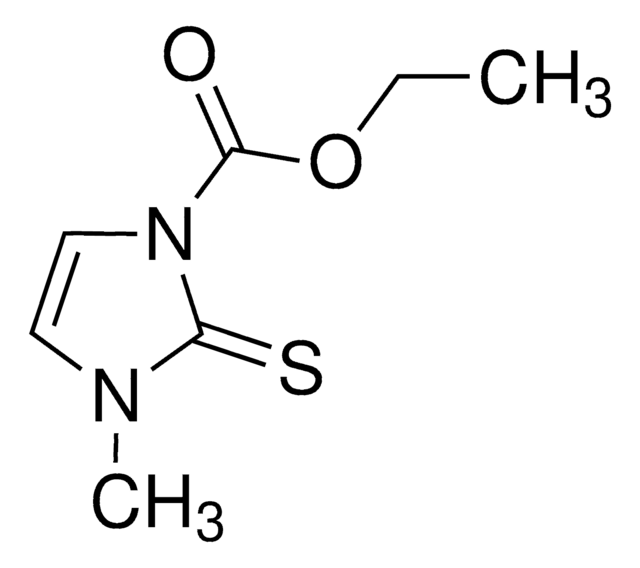
S=[c]1[n](cc[n]1C(=O)OCC)C
InChI
1S/C7H10N2O2S/c1-3-11-7(10)9-5-4-8(2)6(9)12/h4-5H,3H2,1-2H3
InChI Key
CFOYWRHIYXMDOT-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Carbimazole is an orally administered thioimidazole drug, which is indicated for the management of hyperthyroidism as well as Grave′s disease in humans. It is used to facilitate the growth of animals for human consumption.
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
アプリケーション
Carbimazole may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
アナリシスノート
このような2次標準は、入手可能な場合にはUSP、EP、BPの1次標準にマルチトレーサビリティを提供します。
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAA6300 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
おすすめ製品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1684-1G:
PHR1684-1G-PW:
最新バージョンのいずれかを選択してください:
Study of the British Pharmacopeia method on methimazole (thiamazole) content in carbimazole tablets
Aletrari M, et al.
Journal of Pharmaceutical and Biomedical Analysis, 16(5), 785-792 (1998)
Determination of methimazole and carbimazole by flow-injection with chemiluminescence detection based on the inhibition of the Cu (II)-catalysed luminol-hydrogen peroxide reaction
Economou A, et al.
Analytica Chimica Acta, 505(1), 129-133 (2004)
Chromatographic methods development, validation and degradation characterization of the antithyroid drug Carbimazole
Abdelrahman MM
Biomedical Chromatography, 33(4), e4472-e4472 (2019)
HPLC and GC?MS screening of Chinese proprietary medicine for undeclared therapeutic substances.
Liu SY, et al.
Journal of Pharmaceutical and Biomedical Analysis, 24(5-6), 983-992 (2001)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)