おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
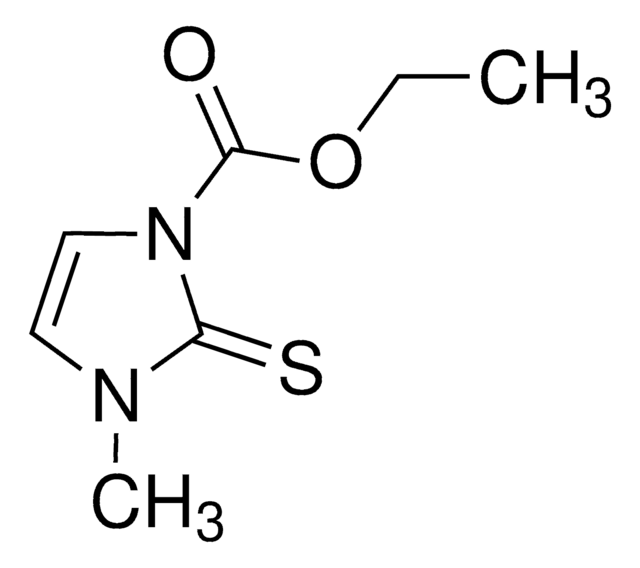
carbimazole
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
InChI
1S/C7H10N2O2S/c1-3-11-7(10)9-5-4-8(2)6(9)12/h4-5H,3H2,1-2H3
InChI Key
CFOYWRHIYXMDOT-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Carbimazole EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Adoree Yi Ying Lim et al.
Singapore medical journal, 54(7), e133-e136 (2013-08-01)
A 24-year-old Chinese woman with Graves' disease presented with myositis two months after treatment with carbimazole. The patient's myositis resolved with hydration and cessation of carbimazole. No other causes of myositis were found, and a change in the medication to
Matteo Cassina et al.
Birth defects research. Part A, Clinical and molecular teratology, 94(8), 612-619 (2012-04-19)
Clinical hyperthyroidism has been associated with an increased risk of maternal, fetal, and neonatal complications. The available antithyroid drugs are methimazole/carbimazole and propylthiouracil. Several case reports and some epidemiologic studies suggest that methimazole/carbimazole exposure during the first trimester of pregnancy
Kirsten Campbell et al.
Australian family physician, 41(8), 564-572 (2012-11-13)
Thyrotoxicosis is common in the Australian community and is frequently encountered in general practice. Graves disease, toxic multinodular goitre, toxic adenoma and thyroiditis account for most presentations of thyrotoxicosis. This article outlines the clinical presentation and evaluation of a patient
[59 year-old man with skin and eye alterations and diabetes mellitus].
N Ewald et al.
Deutsche medizinische Wochenschrift (1946), 137(19), 997-998 (2012-05-03)
Maternal thyrotoxicosis and fetal goitre requiring in utero therapy for hypothyroidism and subsequent neonatal therapy for hyperthyroidism.
Katie Groom et al.
Journal of paediatrics and child health, 49(1), E102-E103 (2013-01-17)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)