おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. Y0000655
traceable to USP 1618003
APIファミリー
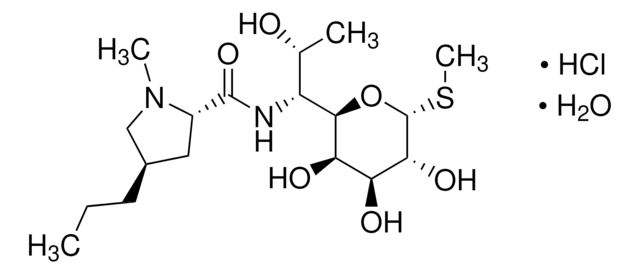
spectinomycin
CofA
current certificate can be downloaded
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
InChI
1S/C14H24N2O7.H2O4S.4H2O/c1-5-4-6(17)14(20)13(21-5)22-12-10(19)7(15-2)9(18)8(16-3)11(12)23-14;1-5(2,3)4;;;;/h5,7-13,15-16,18-20H,4H2,1-3H3;(H2,1,2,3,4);4*1H2/t5-,7-,8+,9+,10+,11-,12-,13+,14+;;;;;/m1...../s1
InChI Key
OBZDRKHRQYPQDZ-SACNDDTHSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Spectinomycin is an aminoglycoside-like antibiotic produced by Streptomyces spectabilis. It is widely used as a veterinary medication to treat gastrointestinal and respiratory infections caused by a variety of spectinomycin-sensitive micro-organisms.
アプリケーション
Spectinomycin Sulfate may be used as a pharmaceutical reference standard for the determination of the analyte in spectinomycin formulations using high-performance liquid chromatography technique.
Spectinomycin is an aminocyclitol antibiotic derived from Streptomyces spectabilis. It has been shown to have a wide variety of uses, including treating acute gonorrheal urethritis and cervictis, to mark cell layers to monitor cell fate during leaf development, as a selection marker in plant related transformation systems for plant cells containing the marker gene Spcr, to study respiratory tract infections of cattle caused by Pasteurella multocida and Mannheimia haemolytica, and to generate plants deficient for the plastid-encoded RNA polymerase on MS-agar media. Spectinomycin also has use in amplification of low copy number plasmid carrying replicons. It is recommended for use in cell culture applications at 7.5-20 mg/L.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
注意
Solutions can be stored at 2-8°C for several weeks or at -20°C for more extended periods.
調製ノート
Stock solutions should be prepared at 10 mg/mL in water and filter sterilized.
脚注
To see an example of a Certificate of Analysis for this material enter LRAB3670 in the slot below. This is an example certificate only and may not be the lot that you receive.
法的情報
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards. These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available. This product was designed, produced and verified for accuracy and stability in accordance with ISO/IEC 17025:2005 (AClass Cert AT-1467), ISO GUIDE 34:2009 (AClass Cert AR-1470).
関連製品
製品番号
詳細
価格
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
この製品を見ている人はこちらもチェック
A validated stability-indicating HPLC method for routine analysis of an injectable lincomycin and spectinomycin formulation
Abualhasan NM, et al.
Scientia Pharmaceutica, 80(4), 977-986 (2012)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)