PHR1428
HEPES
certified reference material,pharmaceutical secondary standard
別名:
4-(2-ヒドロキシエチル)ピペラジン-1-エタンスルホン酸, N-(2-ヒドロキシエチル)ピペラジン-N′-(2-エタンスルホン酸)
About This Item
おすすめの製品
product name
HEPES, Pharmaceutical Secondary Standard; Certified Reference Material
グレード
certified reference material
pharmaceutical secondary standard
品質水準
APIファミリー
hepes
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
pH
5.0-6.5 (25 °C, 238 g/L)
有効pH範囲
6.8-8.2
pKa (25 °C)
7.5
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-30°C
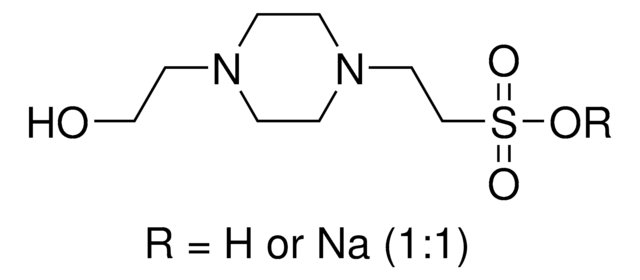
SMILES記法
OCCN1CCN(CC1)CCS(O)(=O)=O
InChI
1S/C8H18N2O4S/c11-7-5-9-1-3-10(4-2-9)6-8-15(12,13)14/h11H,1-8H2,(H,12,13,14)
InChI Key
JKMHFZQWWAIEOD-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
アプリケーション
- HEPES has been used in a wide variety of applications, including tissue culture.
- It is used to buffer cell culture media in air.
- Finds its usage in invitro experiments on Mg.
- Recognized as one of Dr.Good′s recommended buffers, used in some cell culture media as a buffering agent and also in various biochemical reactions.
- Recently, in the production of radiopharmaceuticals, it is the buffer of choice for scientific labeling.
HEPES may also be used as:
- Component of homogenization buffer to homogenize adipose tissue for liquid chromatography/mass spectrometry and in HN buffer for dissolving viral stock titer after dot blot hybridization.
- Pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by high performance liquid chromatography.
アナリシスノート
その他情報
脚注
保管分類コード
13 - Non Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1428-1G:
PHR1428-1G-PW:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)