おすすめの製品
リコンビナント
expressed in CHO cells
品質水準
アッセイ
>90% (SDS-PAGE)
フォーム
liquid
比活性
>1,300 pmol/min-μg
含まれません
preservative
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
avoid repeated freeze/thaw cycles
輸送温度
wet ice
保管温度
−70°C
詳細
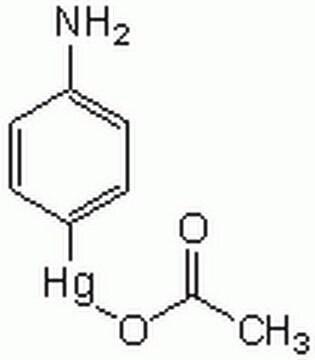
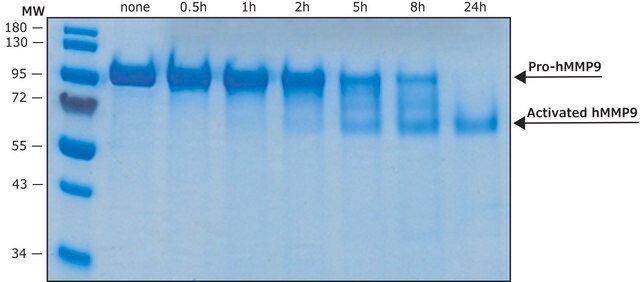
Recombinant, human pro-MMP-9 expressed in CHO cells. The calculated molecular weight is ~77 kDa, but the apparent molecular weight is ~92 kDa by SDS-PAGE. Useful for immunoblotting, substrate cleavage assays, and zymography. MMP-9 Proenzyme can be measured by its ability to degrade gelatin in a zymogram. 0.5 ng of enzyme is sufficient to visualize degraded gelatin with coomassie blue stain. Matrix metalloproteinases are members of a unique family of proteolytic enzymes that have a zinc ion at their active sites and can degrade collagens, elastin and other components of the extracellular matrix (ECM). These enzymes are present in normal healthy individuals and have been shown to have an important role in processes such as wound healing, pregnancy, and bone resorption. However, overexpression and activation of MMPs have been linked with a range of pathological processes and disease states involved in the breakdown and remodeling of the ECM. Such diseases include tumor invasion and metastasis, rheumatoid arthritis, periodontal disease and vascular processes such as angiogenesis, intimal hyperplasia, atherosclerosis and aneurysms. Recently, MMPs have been linked to neurodegenerative diseases such as Alzheimer’s, and amyotrophic lateral sclerosis (ALS). Natural inhibitors of MMPs, tissue inhibitor of matrix metalloproteinases (TIMPs) exist and synthetic inhibitors have been developed which offer hope of new treatment options for these diseases. Regulation of MMP activity can occur at the level of gene expression, including transcription and translation, level of activation, or at the level of inhibition by TIMPs. Thus, perturbations at any of these points can theoretically lead to alterations in ECM turnover. Expression is under tight control by pro- and anti-inflammatory cytokines and/or growth factors and, once produced the enzymes are usually secreted as inactive zymograms. Upon activation (removal of the inhibitory propeptide region of the molecules) MMPs are subject to control by locally produced TIMPs. All MMPs can be activated in vitro with organomercurial compounds (e.g., 4-aminophenylmercuric acetate), but the agents responsible for the physiological activation of all MMPs have not been clearly defined. Numerous studies indicate that members of the MMP family have the ability to activate one another. The activation of the MMPs in vivo is likely to be a critical step in terms of their biological behavior, because it is this activation that will tip the balance in favor of ECM degradation. The hallmark of diseases involving MMPs appear to be stoichiometric imbalance between active MMPs and TIMPs, leading to excessive tissue disruption and often degradation. Determination of the mechanisms that control this imbalance may open up some important therapeutic options of specific enzyme inhibitors.
アプリケーション
Immunoblotting (1 µg protein/lane)
Substrate Cleavage Assay (1 µg protein/lane, see application references)
Zymography (1 µg protein/lane, see application references)
Substrate Cleavage Assay (1 µg protein/lane, see application references)
Zymography (1 µg protein/lane, see application references)
包装
Please refer to vial label for lot-specific concentration.
警告
Toxicity: Standard Handling (A)
単位の定義
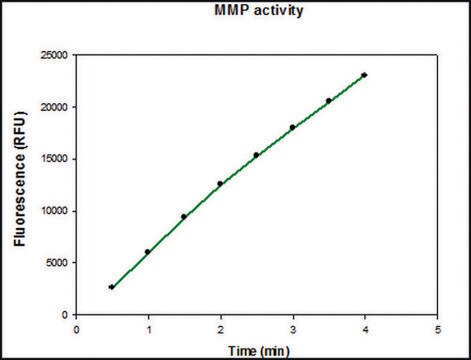
Specific activity is determined using 10 μM (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-Ala-Arg-NH₂ (excitation 320 nm, emission 405 nm), and 20 ng enzyme in 100 μl of 50 mM Tris-HCl, pH 7.5, 10 mM CaCl₂, 150 mM NaCl, and 0.05% BRIJ®-35 Detergent at room temperature.
物理的形状
In 150 mM NaCl, 50 mM Tris-HCl, 10 mM CaCl₂, 0.05% BRIJ®-35 Detergent, pH 7.5.
その他情報
MMP-9 Proenzyme can be measured by its ability to degrade gelatin in a zymogram. 0.5 ng of enzyme is sufficient to visualize degraded gelatin with coomassie blue stain. The specific activity as measured with 10 µM (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-Ala-Arg-NH2 (excitation 320 nm, emission 405 nm), and 20 ng enzyme in 100 µl of 50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 150 mM NaCl, and 0.05% Brij-35 at room temperature, is >1,300 pmoles/min/µg. To activate MMP-9 proenzyme, prepare p-aminophenylmercuric acetate (APMA) concentrate in dimethylsulfoxide (DMSO). Add APMA to MMP-9 proenzyme to give a final APMA concentration of 1 mM. Incubate at 37°C for 16 to 24 h.
Parsons, S.L., et al. 1997. Br. J. Surg.84, 160.
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J Neurochem.67, 251.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta.1251, 99.
Kenagy, R.D. and Clowes, A.W. 1994. in Inhibition of Matrix Metalloproteinases: Therapeutic Potential. Greenwald, R.A. and Golub L.M., Eds.: 462-465.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 209.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 474.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Delaisse, J-M. and Vaes, G. 1992. in Biology and Physiology of the Osteoclast. B.R. Rifkin & C.V. Gay, Eds.: 290-314.
Jeffrey, J.J. 1992. in Wound Healing: Biochemical and Clinical Aspects. R.F. Diegelmann and W.J. Lindblad, Eds.: 177-194.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J Neurochem.67, 251.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta.1251, 99.
Kenagy, R.D. and Clowes, A.W. 1994. in Inhibition of Matrix Metalloproteinases: Therapeutic Potential. Greenwald, R.A. and Golub L.M., Eds.: 462-465.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 209.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 474.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Delaisse, J-M. and Vaes, G. 1992. in Biology and Physiology of the Osteoclast. B.R. Rifkin & C.V. Gay, Eds.: 290-314.
Jeffrey, J.J. 1992. in Wound Healing: Biochemical and Clinical Aspects. R.F. Diegelmann and W.J. Lindblad, Eds.: 177-194.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
法的情報
Brij is a registered trademark of Croda International PLC
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
10 - Combustible liquids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PF038-10UG:
PF038-UG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
Jasper B van Praagh et al.
Surgical infections, 21(10), 865-870 (2020-04-21)
Background: It is now well established that microbes play a key and causative role in the pathogenesis of anastomotic leak. Yet, in patients, determining whether a cultured pathogen retrieved from an anastomotic leak site is a cause or a consequence
M T Kato et al.
Caries research, 44(3), 309-316 (2010-06-17)
It is known that some metal salts can inhibit matrix metalloproteinase (MMP) activity, but the effect of iron has not been tested yet. On the other hand, it has recently been suggested that MMP inhibition might influence dentine erosion. Based
Jose L Orgaz et al.
Nature communications, 5, 4255-4255 (2014-06-26)
Rounded-amoeboid cancer cells use actomyosin contractility driven by Rho-ROCK and JAK-STAT3 to migrate efficiently. It has been suggested that rounded-amoeboid cancer cells do not require matrix metalloproteinases (MMPs) to invade. Here we compare MMP levels in rounded-amoeboid and elongated-mesenchymal melanoma
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)