おすすめの製品
化学種の反応性
rat, mouse, human
メーカー/製品名
Chemicon®
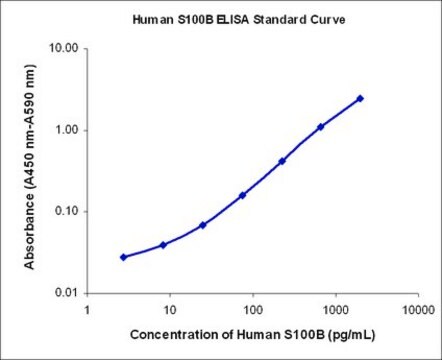
assay range
sensitivity: 1.5 ng/mL
standard curve range: 1.5-100 ng/mL
テクニック
ELISA: suitable
NCBIアクセッション番号
UniProtアクセッション番号
検出方法
colorimetric
遺伝子情報
human ... GFAP(2670)
詳細
Background
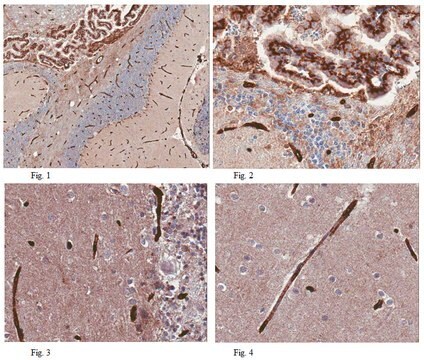
Glial Fibrillary Acidic Protein (GFAP) is a class-III intermediate filament uniquely and ubiquitously expressed as part of the structural network in astrocytic glial cells. This specific expression makes GFAP ideal as a broad astrocytic marker in vertebrates. Astrocytes play a variety of key roles in supporting, guiding, nurturing, and signaling neuronal architecture and activity in a highly interdependent manner. Current understanding of CNS structure suggests that there are at least as many glial cells as the estimated 100 billion neurons, but perhaps upwards of 100 times more. Thus,
the health of both neurons and astrocytes is often affected in disease and trauma. GFAP is proving to be a useful biomarker in the detection of neural degeneration. Although defects in GFAP have been reported to cause certain rare conditions, such as Alexander’s disease (Brenner et al., 2001), the observed interconnected physiology of neuronal and glial cells has enabled the use of GFAP as a neurodegeneration detection gauge. This relationship is being exploited by researchers and clinicians measuring a variety of neurological issues from cancer to trauma. GFAP has been used as a biomarker in the early detection of chemically induced cancer (Capo et al., 1997) and classical glioblastomas (Jung et al., 2007). Elevated levels of GFAP have been reported in traumatic brain injury (TBI) and appear to directly correlate with the severity of injury and brain tissue oxygenation in combat casualty care research (CCR, 2008) as well as civilian accidents (Mondello et al., 2010). GFAP has also been identified as a good biomarker in studies of toxin induced acute brain injury (Liao et al., 2008), in differentiation of hemorrhagic vs. ischemic strokes (Foerch et al., 2006), as well as in cardiac arrest (Kaneko et al., 2009). Millipore now offers an ELISA assay for sensitive measurement of GFAP protein in tissue homogenates and biological fluids (serum, plasma, CSF, and serum free media) from human, mouse, and rat.
Principals of Procedure
This assay is a Sandwich ELISA based sequentially on: 1) capture of soluble GFAP protein to the wells of a microtiter plate coated by a pre-titered amount of anti-GFAP monoclonal antibody; 2) removal of unbound material by washing; 3) binding of biotinylated anti-GFAP monoclonal antibody to
the previously captured GFAP protein; 4) removal of unbound material by washing; 5) binding of streptavidin conjugated horseradish peroxidase to the biotinylated antibodies; 6) removal of excess or unbound enzyme conjugates by washing; and 7) quantification of immobilized antibody-enzyme
complexes by monitoring horseradish peroxidase activity in the presence of the substrate 3,3’,5,5’-tetramethylbenzidine (TMB). Enzyme activity is measured spectrophotometrically by recording absorbance readings at 450 nm. Increases in absorbency are directly proportional to the amount of
GFAP protein captured from the sample. Therefore the protein concentration of unknowns can be derived by interpolation using a standard curve generated in the same assay with reference standards of known concentrations of GFAP.
Glial Fibrillary Acidic Protein (GFAP) is a class-III intermediate filament uniquely and ubiquitously expressed as part of the structural network in astrocytic glial cells. This specific expression makes GFAP ideal as a broad astrocytic marker in vertebrates. Astrocytes play a variety of key roles in supporting, guiding, nurturing, and signaling neuronal architecture and activity in a highly interdependent manner. Current understanding of CNS structure suggests that there are at least as many glial cells as the estimated 100 billion neurons, but perhaps upwards of 100 times more. Thus,
the health of both neurons and astrocytes is often affected in disease and trauma. GFAP is proving to be a useful biomarker in the detection of neural degeneration. Although defects in GFAP have been reported to cause certain rare conditions, such as Alexander’s disease (Brenner et al., 2001), the observed interconnected physiology of neuronal and glial cells has enabled the use of GFAP as a neurodegeneration detection gauge. This relationship is being exploited by researchers and clinicians measuring a variety of neurological issues from cancer to trauma. GFAP has been used as a biomarker in the early detection of chemically induced cancer (Capo et al., 1997) and classical glioblastomas (Jung et al., 2007). Elevated levels of GFAP have been reported in traumatic brain injury (TBI) and appear to directly correlate with the severity of injury and brain tissue oxygenation in combat casualty care research (CCR, 2008) as well as civilian accidents (Mondello et al., 2010). GFAP has also been identified as a good biomarker in studies of toxin induced acute brain injury (Liao et al., 2008), in differentiation of hemorrhagic vs. ischemic strokes (Foerch et al., 2006), as well as in cardiac arrest (Kaneko et al., 2009). Millipore now offers an ELISA assay for sensitive measurement of GFAP protein in tissue homogenates and biological fluids (serum, plasma, CSF, and serum free media) from human, mouse, and rat.
Principals of Procedure
This assay is a Sandwich ELISA based sequentially on: 1) capture of soluble GFAP protein to the wells of a microtiter plate coated by a pre-titered amount of anti-GFAP monoclonal antibody; 2) removal of unbound material by washing; 3) binding of biotinylated anti-GFAP monoclonal antibody to
the previously captured GFAP protein; 4) removal of unbound material by washing; 5) binding of streptavidin conjugated horseradish peroxidase to the biotinylated antibodies; 6) removal of excess or unbound enzyme conjugates by washing; and 7) quantification of immobilized antibody-enzyme
complexes by monitoring horseradish peroxidase activity in the presence of the substrate 3,3’,5,5’-tetramethylbenzidine (TMB). Enzyme activity is measured spectrophotometrically by recording absorbance readings at 450 nm. Increases in absorbency are directly proportional to the amount of
GFAP protein captured from the sample. Therefore the protein concentration of unknowns can be derived by interpolation using a standard curve generated in the same assay with reference standards of known concentrations of GFAP.
アプリケーション
Research Category
ニューロサイエンス
ニューロサイエンス
Used to detect/quantify: GFAP
包装
96 wells
構成
96-Well Anti-GFAP Coated Plate (CS206352): 1 Plate
1X Assay Buffer (CS206357): 40 mL
10X Wash Buffer (CS206360): 50 mL
GFAP Protein Standard (CS206361): 1 Vial
GFAP Quality Control #1 (CS206351): 1 Vial
GFAP Quality Control #2 (CS206475): 1 Vial
Biotinylated Anti-GFAP Detection Antibody (CS206356): 12 mL
Enzyme Solution (CS206358): 12 mL
TMB Solution (CS202682): 10 mL
Stop Solution (60193): 12 mL
Plate Sealers (CS200443): 10 Sheets
1X Assay Buffer (CS206357): 40 mL
10X Wash Buffer (CS206360): 50 mL
GFAP Protein Standard (CS206361): 1 Vial
GFAP Quality Control #1 (CS206351): 1 Vial
GFAP Quality Control #2 (CS206475): 1 Vial
Biotinylated Anti-GFAP Detection Antibody (CS206356): 12 mL
Enzyme Solution (CS206358): 12 mL
TMB Solution (CS202682): 10 mL
Stop Solution (60193): 12 mL
Plate Sealers (CS200443): 10 Sheets
保管および安定性
Storage: The GFAP ELISA kit is shipped on ice and should be stored at 2-8°C upon arrival.
Stability: Kit components are stable for a minimum of 6 months from the date of receipt if stored and
handled as described above.
Stability: Kit components are stable for a minimum of 6 months from the date of receipt if stored and
handled as described above.
法的情報
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
免責事項
For research use only. Not for use in diagnostic procedures.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Aquatic Chronic 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Sens. 1
保管分類コード
8A - Combustible corrosive hazardous materials
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
NS830:
NS830-B:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)