おすすめの製品
由来生物
mouse
品質水準
抗体製品の状態
purified immunoglobulin
抗体製品タイプ
primary antibodies
クローン
12D10, monoclonal
化学種の反応性
human
化学種の反応性(ホモロジーによる予測)
all
テクニック
flow cytometry: suitable
immunocytochemistry: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
アイソタイプ
IgG2aκ
輸送温度
wet ice
ターゲットの翻訳後修飾
unmodified
遺伝子情報
human ... NPEPPS(9520)
関連するカテゴリー
詳細
Puromycin is an aminonucleoside antibiotic, derived from the Streptomyces alboniger bacterium, that functions as a protein synthesis inhibitor that blocks translation through premature chain termination in the ribosome. Monoclonal antibodies to puromycin may be used with standard immunochemical methods to directly monitor translation, a method known as surface sensing of translation (SUnSET). Part of the molecule resembles the 3′ end of the aminoacylated tRNA, making it useful for protein translation analysis. Puromycin induces DNA fragmentation in thymocytes and in human HL-60 leukemia cells.
特異性
Anti-Puromycin Antibody, clone 12D10 demonstrated to react with Human test sample, preincubated with Puromycin. Predicted to react with all species when test sample is incubated with Puromycin.
免疫原
Puromycin from Streptomyces alboniger
アプリケーション
Research Category
エピジェネティクス及び核内機能分子
エピジェネティクス及び核内機能分子
Research Sub Category
RNA代謝及び結合タンパク質
RNA代謝及び結合タンパク質
Anti-Puromycin antibody, clone 12D10, detects puromycin incorporated into protein. Monoclonal antibodies to puromycin may be used with standard immunochemical methods.
Western Blotting Analysis (Total Protein Staining): HEK293 cell lysates treated with Puromycin and Cyclohexamide, or Puromycin only were resolved using SDS-PAGE and transferred to a membrane. Proteins were visualized using Ponceau S staining.
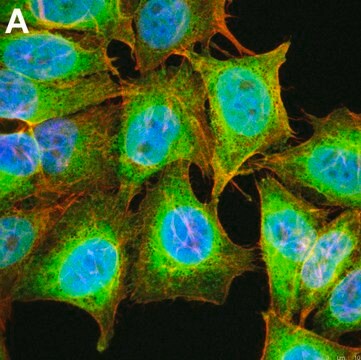
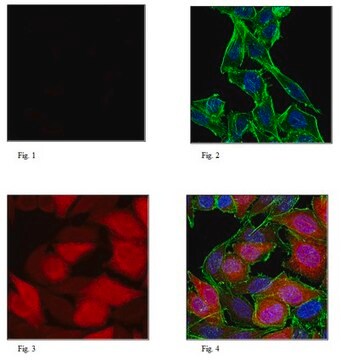
Immunocytochemistry Analysis: A 1:10,000 dilution from a representative lot detected Puromycin-incorporated neosynthesized proteins in HeLa cells treated with Puromycin.
Western Blotting Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in WB (Reineke, L. C., et al. (2012). Mol Biol Cell. 23(18):3499-3510.; Trinh, M. A. et al. (2012). Cell Rep. 1(6):678-688.; Fortin, D. A., et al. (2012). J Neurosci. 32(24):8127-8137.; Clavarino, G., et al. (2012). PLoS Pathog. 8(5):e1002708.; David, A., et al. (2012). J Cell Biol. 197(1):45-57.; White, L. K., et al. (2011). J Virol. 85(1):606-620.; Hoeffer, C. A., et al. (2011). Proc Natl Acad Sci USA. 108(8):3383-3388.; Goodman, C. A., et al. (2010). FASEB J. 25(3):1028-1039.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.; Santini, E., et al. (2013). Nature. 493(7432):411-415.; Quy. P. N., et al. (2013). J Biol Chem. 288(2):1125-1134.; Hulmi. J. J., et al. (2012). Am J Physiol Endocrinol Metab. 304(1):E41-50.; Bhattacharya, A., et al. (2012). Neuron. 76(2):325-337.; Hoeffer, C. A., et al. (2013). J Neurophysiol. 109(1):68-76.).
Immunofluorescence Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in WB (Reineke, L. C., et al. (2012). Mol Biol Cell. 23(18):3499-3510.; Trinh, M. A. et al. (2012). Cell Rep. 1(6):678-688.; Fortin, D. A., et al. (2012). J Neurosci. 32(24):8127-8137.;David, A., et al. (2012). J Cell Biol. 197(1):45-57.; David, A., et al. (2011). J Biol Chem. 286(23):20688-20700.; White, L. K., et al. (2011). J Virol. 85(1):606-620.; Hoeffer, C. A., et al. (2011). Proc Natl Acad Sci USA. 108(8):3383-3388.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.; Goodman, C. A., et al. (2012). Proc Natl Acad Sci USA. 109(17):E989.; Santini, E., et al. (2013). Nature. 493(7432):411-415.; Quy. P. N., et al. (2013). J Biol Chem. 288(2):1125-1134.).
Immunohistochemistry Analysis: A representative lot detecte Puromycin-incorporated neosynthesized protein in IHC (Goodman, C. A., et al. (2010). FASEB J. 25(3):1028-1039.).
Fluorescence Activated Cell Sorting Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in FACS (David, A., et al. (2012). J Cell Biol. 197(1):45-57.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.).
Alexa Fluor™ is a registered trademark of Life Technologies.
Immunocytochemistry Analysis: A 1:10,000 dilution from a representative lot detected Puromycin-incorporated neosynthesized proteins in HeLa cells treated with Puromycin.
Western Blotting Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in WB (Reineke, L. C., et al. (2012). Mol Biol Cell. 23(18):3499-3510.; Trinh, M. A. et al. (2012). Cell Rep. 1(6):678-688.; Fortin, D. A., et al. (2012). J Neurosci. 32(24):8127-8137.; Clavarino, G., et al. (2012). PLoS Pathog. 8(5):e1002708.; David, A., et al. (2012). J Cell Biol. 197(1):45-57.; White, L. K., et al. (2011). J Virol. 85(1):606-620.; Hoeffer, C. A., et al. (2011). Proc Natl Acad Sci USA. 108(8):3383-3388.; Goodman, C. A., et al. (2010). FASEB J. 25(3):1028-1039.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.; Santini, E., et al. (2013). Nature. 493(7432):411-415.; Quy. P. N., et al. (2013). J Biol Chem. 288(2):1125-1134.; Hulmi. J. J., et al. (2012). Am J Physiol Endocrinol Metab. 304(1):E41-50.; Bhattacharya, A., et al. (2012). Neuron. 76(2):325-337.; Hoeffer, C. A., et al. (2013). J Neurophysiol. 109(1):68-76.).
Immunofluorescence Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in WB (Reineke, L. C., et al. (2012). Mol Biol Cell. 23(18):3499-3510.; Trinh, M. A. et al. (2012). Cell Rep. 1(6):678-688.; Fortin, D. A., et al. (2012). J Neurosci. 32(24):8127-8137.;David, A., et al. (2012). J Cell Biol. 197(1):45-57.; David, A., et al. (2011). J Biol Chem. 286(23):20688-20700.; White, L. K., et al. (2011). J Virol. 85(1):606-620.; Hoeffer, C. A., et al. (2011). Proc Natl Acad Sci USA. 108(8):3383-3388.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.; Goodman, C. A., et al. (2012). Proc Natl Acad Sci USA. 109(17):E989.; Santini, E., et al. (2013). Nature. 493(7432):411-415.; Quy. P. N., et al. (2013). J Biol Chem. 288(2):1125-1134.).
Immunohistochemistry Analysis: A representative lot detecte Puromycin-incorporated neosynthesized protein in IHC (Goodman, C. A., et al. (2010). FASEB J. 25(3):1028-1039.).
Fluorescence Activated Cell Sorting Analysis: A representative lot detected Puromycin-incorporated neosynthesized proteins in FACS (David, A., et al. (2012). J Cell Biol. 197(1):45-57.; Schmidt, E., K., et al. (2009). Nat Methods. 6(4):275-277.).
Alexa Fluor™ is a registered trademark of Life Technologies.
品質
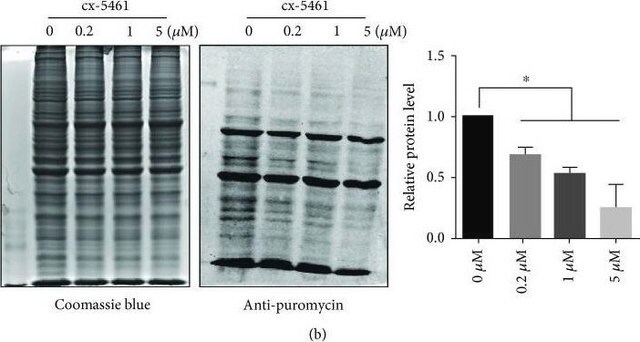
Evaluated by Western Blotting in HEK293 cell lysates treated with Puromycin and Cyclohexamide, or with Puromycin only.
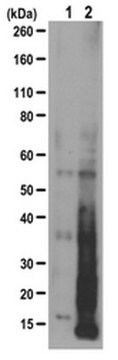
Western Blotting Analysis: A 1:25,000 dilution of this antibody detected Puromycin-incorporated neosynthesized proteins in HEK293 cell lysates treated with Puromycin only. This antibody also detected small mounts of Puromycin-incorporated neosynthesized proteins in HEK293 cells treated with Puromycin and Cyclohexamide.
Western Blotting Analysis: A 1:25,000 dilution of this antibody detected Puromycin-incorporated neosynthesized proteins in HEK293 cell lysates treated with Puromycin only. This antibody also detected small mounts of Puromycin-incorporated neosynthesized proteins in HEK293 cells treated with Puromycin and Cyclohexamide.
ターゲットの説明
Puromycin is incorporated in neosynthesized proteins. In the presense of Puromycin only, this antibody detects Puromycin-incorporated neosynthesized proteins at multiple molecular weights. However, a weaker signal is observed in the co-presense of Cycloheximide, an inhibitor of protein biosynthesis in eukaryotic organisms.
物理的形状
Protein G Purified
Format: Purified
Purified mouse monoclonal IgG2aκ in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide.
保管および安定性
Stable for 1 year at 2-8°C from date of receipt.
アナリシスノート
Control
HEK293 cell lysates treated with Puromycin and Cyclohexamide, or with Puromycin only.
HEK293 cell lysates treated with Puromycin and Cyclohexamide, or with Puromycin only.
その他情報
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
法的情報
ALEXA FLUOR is a trademark of Life Technologies
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our 製品選択ツール.
保管分類コード
12 - Non Combustible Liquids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
Alexandre David et al.
The Journal of cell biology, 197(1), 45-57 (2012-04-05)
Whether protein translation occurs in the nucleus is contentious. To address this question, we developed the ribopuromycylation method (RPM), which visualizes translation in cells via standard immunofluorescence microscopy. The RPM is based on ribosome-catalyzed puromycylation of nascent chains immobilized on
Chikungunya virus induces IPS-1-dependent innate immune activation and protein kinase R-independent translational shutoff.
White, LK; Sali, T; Alvarado, D; Gatti, E; Pierre, P; Streblow, D; Defilippis, VR
Journal of virology null
SUnSET, a nonradioactive method to monitor protein synthesis.
Schmidt, Enrico K, et al.
Nature Methods, 6, 275-277 (2009)
Imaging of protein synthesis with puromycin.
Goodman, Craig A, et al.
Proceedings of the National Academy of Sciences of the USA, 109, E989-E989 (2012)
Craig A Goodman et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25(3), 1028-1039 (2010-12-15)
In this study, the principles of surface sensing of translation (SUnSET) were used to develop a nonradioactive method for ex vivo and in vivo measurements of protein synthesis (PS). Compared with controls, we first demonstrate excellent agreement between SUnSET and
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)