362330
GAG Antagonist, Surfen
The GAG Antagonist, Surfen controls the biological activity of GAG. This small molecule/inhibitor is primarily used for Activators/Inducers applications.
別名:
GAG Antagonist, Surfen, Gβγ Activator, 12155, NSC12155, bis-2-Methyl-4-amino-quinolyl-6-carbamide, diHCl, trihydrate, Glycosaminoglycans Antagonist
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
実験式(ヒル表記法):
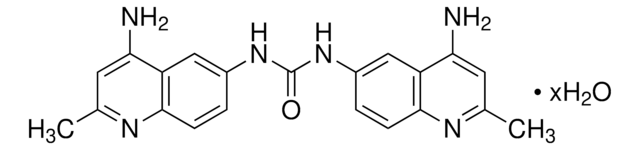
C21H20N6O · 2HCl · 3H2O
分子量:
499.39
UNSPSCコード:
12352200
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
desiccated (hygroscopic)
protect from light
色
white
溶解性
DMSO: 50 mg/mL
輸送温度
ambient
保管温度
2-8°C
詳細
A symmetrical quinolyl-urea compound that binds GAGs (glycosaminoglycans) via electrostatic interaction with the negatively charged sulfate and carboxyl moieties present in heparan sulfate (HS), heparin, and dermatan sulfate, resulting in effective blockage of GAGs interactions with their protein binding partners. Surfen is shown to effectively neutralize HS- and heparin-mediated thrombin inhibition of Factor Xa activity as well as heparin′s anti-clotting activity. Also reported to inhibit FGF2-induced Erk phosphorylation and tubulation in murine lung endothelial cultures (IC50 ~5 µM), fibronectin HS-binding domain-dependent CHO cell adhesion (IC50 = 3 µM), and HSV-1 infection of glucosaminyl 3-O-sulfotransferase-3A-expressing CHO cells (complete inhibition at 5 µM). Surfen analogs with improved potency may serve as promising candidates as less toxic alternatives to Protamine (Cat. No. 539122) in clinical applications.Reported to directly bind Gβγ subunit in a reversible manner, displace Gα-GDP from Gβγ, and acutely activate Gβγ signaling without Gα activation.
A symmetrical quinolyl-urea compound that binds GAGs (glycosaminoglycans) via electrostatic interaction with the negatively charged sulfate and carboxyl moieties present in heparan sulfate (HS), heparin, and dermatan sulfate, resulting in effective blockage of GAGs interactions with their protein binding partners. Surfen is shown to effectively neutralize HS- and heparin-mediated thrombin inhibition of Factor Xa activity as well as heparin′s anti-clotting activity. Also reported to inhibit FGF2-induced Erk phosphorylation and tubulation in murine lung endothelial cultures (IC50 ~5 µM), fibronectin HS-binding domain-dependent CHO cell adhesion (IC50 = 3 µM), and HSV-1 infection of glucosaminyl 3-O-sulfotransferase-3A-expressing CHO cells (complete inhibition at 5 µM). Surfen analogs with improved potency may serve as promising candidates as less toxic alternatives to Protamine (Cat. No. 539122) in clinical applications.
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
再構成
Following reconstitution, aliquot into glass vials and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
その他情報
Surve, C.R., et al. 2016. Sci. Signal.9, ra22.
Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA105, 13074.
Hunter, D.T. Jr., and Hill, J.M. 1961. Nature191, 1378.
Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA105, 13074.
Hunter, D.T. Jr., and Hill, J.M. 1961. Nature191, 1378.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
362330-100MG:
362330-MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Ferenc Zsila
Biochemical and biophysical research communications, 460(3), 863-867 (2015-04-02)
It is shown that the heparin antagonist bis-aminoquinoline derivative surfen interacts with sulfated cyclodextrins in a unique fashion. Analysis of the UV spectroscopic data revealed exceptionally strong association (K(a) ∼ 10(7) M(-1)) of several surfen molecules to the external surface
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)