1.05454
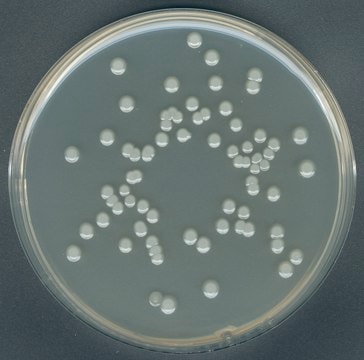
GranuCult® prime BRILA (Brilliant-green bile Lactose) broth
according to ISO 4831:2006, ISO 4832:2006, FDA-BAM, for Escherichia coli, for coliforms, suitable for microbiology
別名:
BGB broth, BGBLB, Brilliant Green Bile broth, Brilliant-green bile lactose broth, Brilliant-green bile lactose broth, Brillant-green bile lactose broth
About This Item
おすすめの製品
認証
SMWW Part 9221
APHA
FDA-BAM
GB 4789.3:2016
ISO 4832:2006
according to ISO 4831:2006
品質水準
形状
medium granules (dehydrated (DCM))
包装
pkg of 5 kg
pkg of 500 g
メーカー/製品名
GranuCult® prime
保管条件
dry media (Keep tightly closed)
テクニック
microbiological culture: suitable
色
green
pH
7.2 (30 °C, 40 g/L in H2O, after autoclaving)
溶解性
40 g/L
かさ密度
560 kg/m3
アプリケーション
food and beverages
microbiology
保管温度
15-25°C
適合性
Escherichia coli
coliforms
関連するカテゴリー
詳細
アプリケーション
特徴および利点
- GranuCult® offers superior granulated culture media
- Safe and sustainable due to reduced risks associated with fine dust and toxic substance inhalation, resulting in a safer work environment
- Excellent wettability, solubility, and free-flowing properties
- Convenient, with minimal component separation and clumping, even under warm or humid conditions
- High batch-to-batch reproducibility
- Prolonged shelf life of up to five years
- High number of test strains exceeding all regulatory demands
- Granulation technology allows many supplements to be included, with no need to add these separately
脚注
The designations basic, plus, or prime are added to indicate the quality control level, from basic quality control to standard QC plus to prime for full regulatory compliance.
法的情報
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
Microbial culture media is available in both powdered and granulated forms. This article compares the characteristics of each culture media format with regards to safety, handling and convenience.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)