おすすめの製品
アッセイ
>99% (HPLC)
形状
powder
包装
pkg of 1 × 1 mg (699852P-1mg)
pkg of 1 × 5 mg (699852P-5mg)
メーカー/製品名
Avanti Research™ - A Croda Brand
アプリケーション
vaccine development
輸送温度
dry ice
保管温度
−20°C
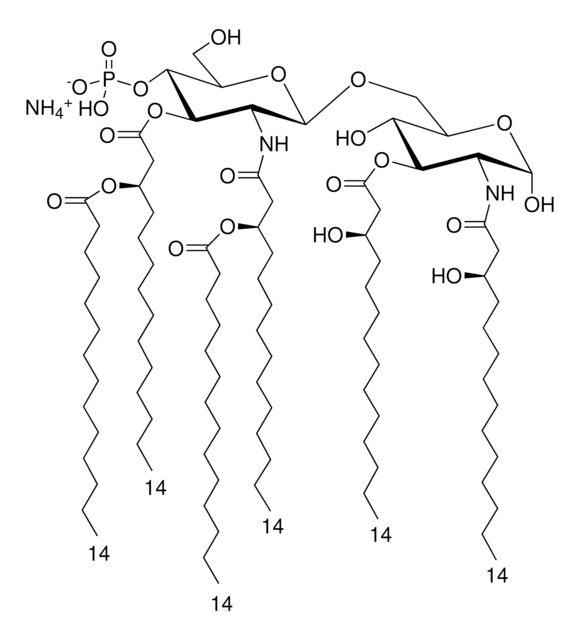
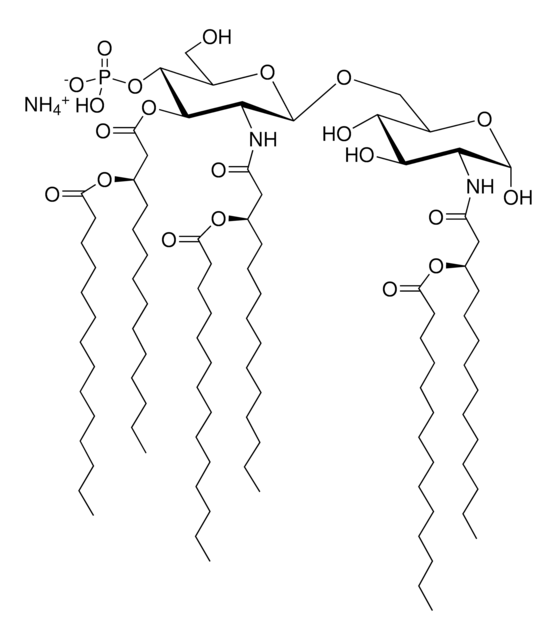
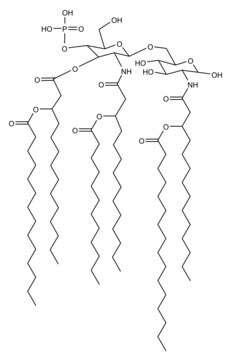
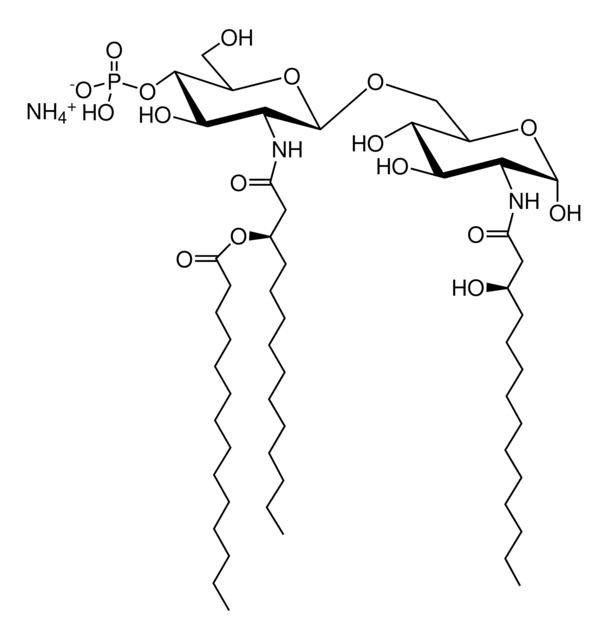
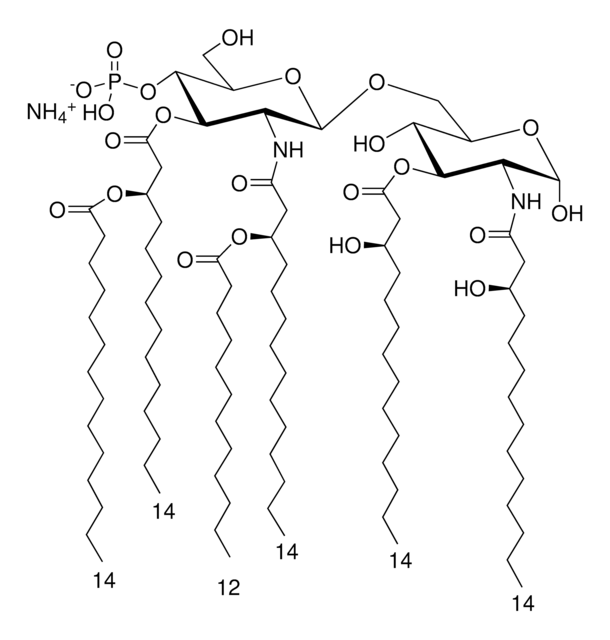
SMILES記法
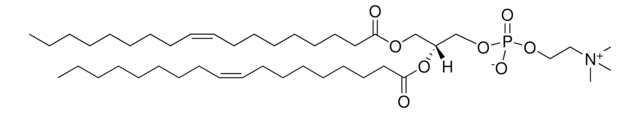
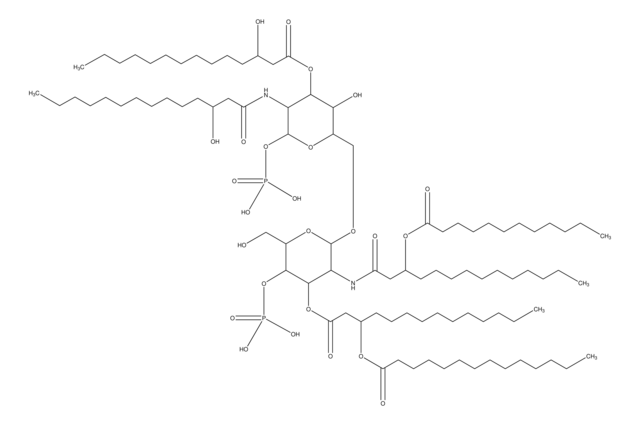
O[C@H]([C@@H](CO[C@@H]([C@@H]1NC(C[C@H](OC(CCCCCCCCCCCCC)=O)CCCCCCCCCCC)=O)O[C@H](CO)[C@@H](OP([O-])(O)=O)[C@@H]1OC(C[C@H](OC(CCCCCCCCCCCCC)=O)CCCCCCCCCCC)=O)O[C@H](O)[C@@H]2NC(C[C@H](O)CCCCCCCCCCC)=O)[C@@H]2O.[NH4+]
詳細
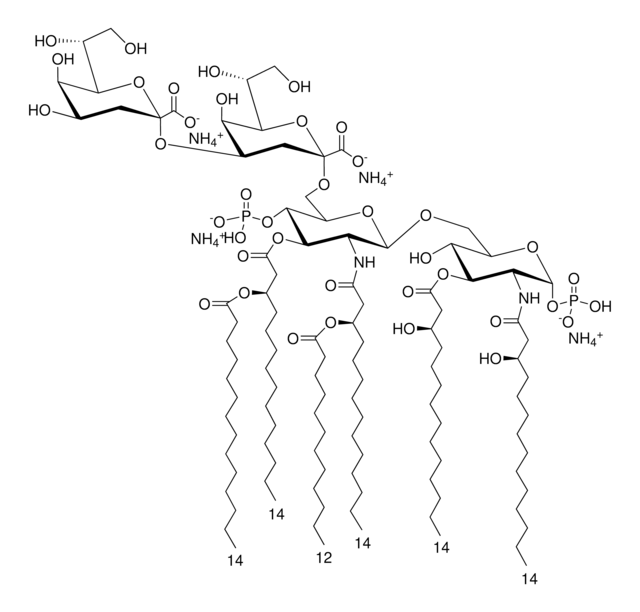
Vaccination is well-accepted as an effective method to prevent infections by mounting pathogen-specific immune responses prior to the infection. Usually, immunization with vaccine antigens alone is not able to induce robust or long-lasting immune responses — resulting in failure of protective immunity against infections. Thus, adjuvants are required to enhance cellular or humoral immune responses upon immunization. Because vaccine adjuvants using Lipid A have proven to be safe and effective in inducing Th-1 type immune responses to heterologous proteins in animal and human vaccines, Avanti developed Phosphorylated HexaAcyl Disaccharide (PHAD™), the first fully synthetic monophosphoryl Lipid A available for use as an adjuvant in human vaccines.
アプリケーション
3D-PHAD™ has been used:
- as a synthetic adjuvant for respiratory syncytial virus (RSV) vaccine
- as a synthetic adjuvant to prepare hepatitis B virus (HBV) and virus-like particles (VLP)-based human papillomavirus (HPV) vaccines
- in liposome preparation for the generation of army liposome formulation (ALF) for human immunodeficiency (HIV) gp140 antigen immunization studies in mice.
生物化学的/生理学的作用
3-deacyl-phosphorylated hexa-acyl disaccharide (3D-PHAD™) is derived from monophosphoryl lipid A. It is amphiphilic and associates with the virosomal membranes.
包装
2 mL Amber Glass Crimp Cap Vial (699852P-1mg)
2 mL Amber Glass Crimp Cap Vial (699852P-5mg)
その他情報
For R&D use only. Not for drug, household, or other uses.
法的情報
3D-PHAD is a trademark of Avanti Polar Lipids, LLC
Avanti Research is a trademark of Avanti Polar Lipids, LLC
PHAD is a trademark of Avanti Polar Lipids, LLC
保管分類コード
11 - Combustible Solids
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
699852P-VAR:
699852P-1MG:
699852P-BULK:
699852P-5MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
Development of a virosomal RSV vaccine containing 3D-PHAD Adjuvant: formulation, composition, and long-term stability
Lederhofer J
Pharmaceutical Research, 35(9), 172-172 (2018)
Monica L Martin et al.
Malaria journal, 18(1), 377-377 (2019-11-30)
Indian-origin rhesus (InR) are preferred for research, but strict export restrictions continue to limit their use. Chinese-origin rhesus (ChR), although easier to procure, are genetically distinct from InR and differ in their immune response to infectious agents, such as the
Immune response to antigen adsorbed to aluminum hydroxide particles: Effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex
Beck Z, et al.
Journal of Controlled Release : Official Journal of the Controlled Release Society, 275, 1219-1219 (2018)
Immunogenicity of HPV and HBV vaccines: adjuvanticity of synthetic analogs of monophosphoryl lipid A combined with aluminum hydroxide
Taleghani N, et al.
Acta Pathologica, Microbiologica et Immunological Scandinavica, 127(3), 150-157 (2019)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)