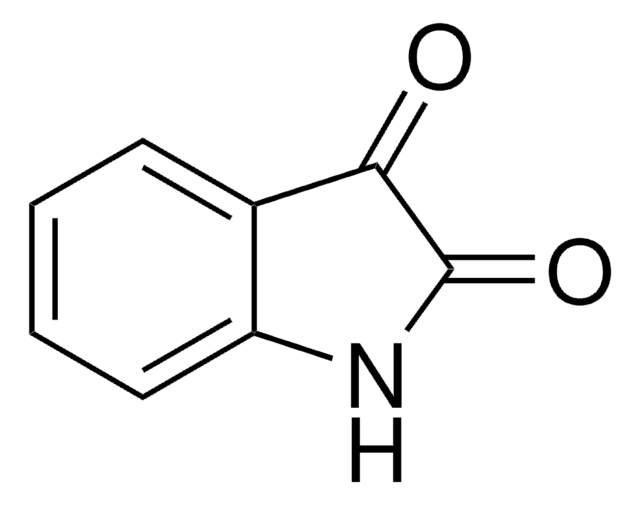
おすすめの製品
蒸気密度
5.6 (vs air)
アッセイ
96%
mp
233 °C (dec.) (lit.)
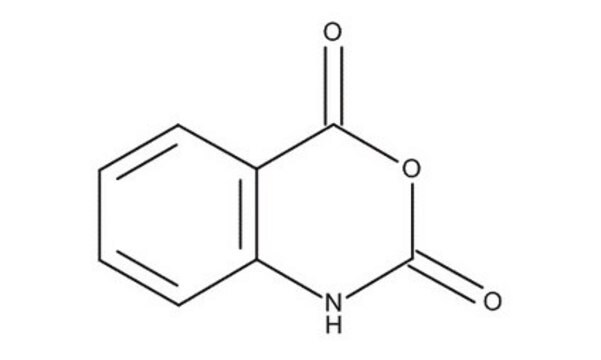
SMILES記法
O=C1Nc2ccccc2C(=O)O1
InChI
1S/C8H5NO3/c10-7-5-3-1-2-4-6(5)9-8(11)12-7/h1-4H,(H,9,11)
InChI Key
TXJUTRJFNRYTHH-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
586.4 °F - closed cup
引火点(℃)
308 °C - closed cup
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
I12808-500G:
I12808-25KG:
I12808-BULK:
I12808-100G:
I12808-5G:
I12808-VAR:
この製品を見ている人はこちらもチェック
Jonathan J Goodall et al.
Chembiochem : a European journal of chemical biology, 3(1), 68-75 (2007-06-27)
The acyl-enzyme formed upon acylation of alpha-chymotrypsin with isatoic anhydride has been characterised by infrared spectroscopy. Acylation at pH 7 to yield the 2-aminobenzoyl-enzyme is rapid (k = 5.57x 10(-2)s(-1)), while deacylation is much slower (k =3.7 x 10(-5)10(-2) (s-).
P S Gravett et al.
The International journal of biochemistry, 23(10), 1101-1110 (1991-01-01)
1. Esterase E-I from Bitis gabonica was inactivated with irreversible inhibitors which included studies with a water-soluble carbodiimide, an affinity labelling peptide and a mechanism-based inactivator. 2. The reaction with 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide was biphasic and the dominant part followed saturation kinetics.
Zheng-Hui Guan et al.
Journal of the American Chemical Society, 134(42), 17490-17493 (2012-10-12)
A Pd-catalyzed regioselective C-H bond carbonylation of N-alkyl anilines for the synthesis of isatoic anhydrides has been developed. The key Pd-catalyst intermediate has been isolated and characterized. This novel Pd-catalyzed carbonylation reaction tolerates a wide range of functional groups and
K Siva Kumar et al.
Organic & biomolecular chemistry, 10(15), 3098-3103 (2012-03-10)
A one-pot cascade reaction has been developed leading to the concurrent construction of six and five membered fused N-heterocyclic rings of indazolo[3,2-b]quinazolinones. The methodology involved the reaction of isatoic anhydride, a hydrazine and o-iodo benzaldehyde in the presence of Pd(PPh(3))(4)
M H Gelb et al.
Journal of medicinal chemistry, 29(4), 585-589 (1986-04-01)
Derivatives of isatoic anhydride were prepared and tested as inhibitors of serine proteases. A number of isatoic anhydrides with positively charged substituents irreversibly inactivated several trypsin-like enzymes and preferentially inactivated trypsin over chymotrypsin. Further selectivity was obtained by introduction of
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)